
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
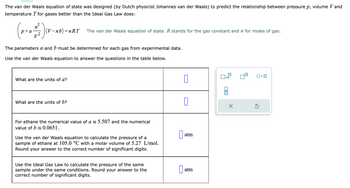
Transcribed Image Text:The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume V and
temperature T for gases better than the Ideal Gas Law does:
n
(p+₁ +²2) (v-
(V-nb)=nRT
The parameters a and b must be determined for each gas from experimental data.
Use the van der Waals equation to answer the questions in the table below.
What are the units of a?
The van der Waals equation of state. R stands for the gas constant and n for moles of gas.
What are the units of b?
For ethane the numerical value of a is 5.507 and the numerical
value of b is 0.0651.
Use the van der Waals equation to calculate the pressure of a
sample of ethane at 105.0 °C with a molar volume of 5.27 L/mol.
Round your answer to the correct number of significant digits.
Use the Ideal Gas Law to calculate the pressure of the same
sample under the same conditions. Round your answer to the
correct number of significant digits.
0
0
atm
atm
x10
DO
X
ロ・ロ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- P1A.12* Balloons are still used to deploy sensors that monitor meteorological phenomena and the chemistry of the atmosphere. It is possible to investigate some of the technicalities of ballooning by using the perfect gas law. Suppose your balloon has a radius of 3.0 m and that it is spherical. (a) What amount of H₂ (in moles) is needed to inflate it to 1.0 atm in an ambient temperature of 25 °C at sea level? (b) What mass can the balloon lift (the payload) at sea level, where the mass density of air is 1.22 kg m? (c) What would be the payload if He were used instead of H₂?arrow_forwardA sample of gas contains 0.2000 mol of CH4(g) and 0.4000 mol of O2(g) and occupies a volume of 24.0 L. The following reaction takes place:CH4(g) + 2O2(g)CO2(g) + 2H2O(g)Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.arrow_forwardSuppose a sample of refrigerant gas consisting of a simple mixture of the gases C2HF5 and CH2F2 has a density of 1.92 g/L at 31 °C and 0.625 atm. 1) Calculate the average molecular mass for this sample. 2) Calculate the volume percentage of CH2F2 in the sample.arrow_forward
- 0.17 moles of hydrogen gas and 0.33 moles of nitrogen gas are created from thereaction 2NH3 (g) → 3H2 (g) + N2 (g). The final total pressure in the reaction vessel is0.75 atm. Calculate the partial pressure of the hydrogen gas at the end of the reaction.Assume the number of moles of NH3 (g), left after the reaction occurs, is negligible.arrow_forwardThe density of a gas is 1.43 g/Lg/L at a temperature of 23 ∘C∘C and a pressure of 0.789 atmatm. Calculate the molar mass of the gas.arrow_forwardSometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. 2 Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 40.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 51.0 mL. g x10 X 歐 Sketch of a gas-collection apparatus S collected gas Calculate the mass of CO₂ that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about 2 the reaction conditions and the nature of the gases. water chemical reactionarrow_forward
- For many purposes we can treat ammonia (NH,) as an ideal gas at temperatures above its boiling point of – 33. °C. Suppose the temperature of a sample of ammonia gas is lowered from 15.0 °C to – 14.0 °C, and at the same time the pressure is increased by 15.0%. increase x10 Does the volume of the sample increase, decrease, or stay the same? decrease ? stays the same If you said the volume increases or decreases, calculate the percentage change in the volume. Round your answer to the nearest percent.arrow_forwardA 9.00 L tank at 19.4 °C is filled with 2.75 g of chlorine pentafluoride gas and 13.9 g of sulfur hexafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the partial pressure of each gas in the tank. Be sure your answers have the correct number of significant digits. chlorine pentafiuoride partial pressure: sulfur hexafluoride partial pressure: ? atmarrow_forwardThe van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume V and temperature T for gases better than the Ideal Gas Law does: The van der Waals equation of state. R stands for the gas constant and n for (V-nb)=nRT moles of gas. The parameters a and b must be determined for each gas from experimental data. Use the van der Waals eguation to answer the questions in the table below. 6. -2 What are the units of a? Ра m mol 몸 3 -1 What are the units of b? m ·mol For ethane the numerical value of a is 5.507 and the numerical value of b is 0.0651. | atm Use the van der Waals equation to calculate the pressure of a sample of ethane at 75.0 °C with a molar volume of 4,10 L/mol. Round your answer to the correct number of significant digits. Use the Ideal Gas Law to calculate the pressure of the same sample under the same conditions. Round your answer to the correct number of significant digits. atmarrow_forward
- A container holds a mixture of gases with the following partial pressures: PO₂ = 40.3 kPa, PCO₂ = 0.89 kPa, PN₂ = 180.3 kPa, and Ptotal = 222.8 kPa. Calculate the pressure of the other gases as described by Dalton's law of partial pressures.arrow_forwardA sample of gas contains 0.1100 mol of CH4 (9) and 0.1100 mol of H₂O(g) and occupies a volume of 13.1 L. The following reaction takes place: CH4 (9) + H₂O(g) → 3H₂(g) + CO(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant. Volume =arrow_forwardA student obtained a mass of 0.266 g CO2 during his experiment. The atmospheric pressure was 751.6 mmHg and the laboratory temperature was 24.1 °C. The flask used in the experiment had a volume of 146.5 mL. Calculate the molar mass of CO2 from the data. Hint: You cannot use the molar mass of CO2 in your calculation since this is what you are looking for... You need to used the ideal gas law to determine the number of moles of CO2, then do the ratio of grams of CO2 over moles of CO2 to determine the experimental molar mass of CO2.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY