Question
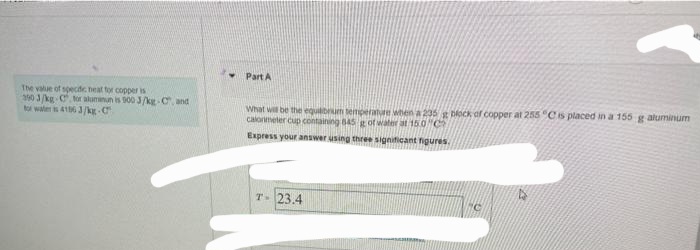
Transcribed Image Text:The value of specific neat for copper is
390 3/kg-C for aluminun is 900 J/kg-C, and
for water is 4156 3/kg-C
Part A
What will be the equilibrum temperature when a 235 g block of copper at 255 °C is placed in a 155 g aluminum
calorimeter cup containing 845 g of water at 15.0 C
Express your answer using three significant figures.
T- 23.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images
