
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:The value of Henry's constant KH is
Select one:
а.
Constant for all gases.
b.
Greater for gases with higher solubility.
c.
Greater for gases with lower solubility.
d.
Not related to the solubility of gases.
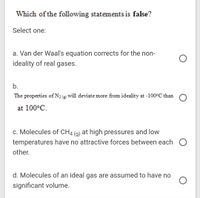
Transcribed Image Text:Which of the following statements is false?
Select one:
a. Van der Waal's equation corrects for the non-
ideality of real gases.
b.
The properties of N2@ will deviate more from ideality at -100°C than O
at 100°C.
c. Molecules of CH4 (g) at high pressures and low
temperatures have no attractive forces between each O
other.
d. Molecules of an ideal gas are assumed to have no
significant volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the ideal gas law to verify the triple product rule.arrow_forwardA gaseous mixture consists of 1.26 mol of helium, 0.36 mol of neon, and 1.58 mol of argon. What is the mole fraction of helium in the mixture?arrow_forwardCalculate the most probable kinetic energy for 2.00 mol of molecular nitrogen at 25°C and 1.0 atm.arrow_forward
- Imagine that a chemist puts 4.33 mol each of C3H8 and O₂ in a 1.00-L container at constant temperature of 505 °C. This reaction occurs: C3H8(g) + 502(g) 3C0₂(g) + 4H₂O(g) When equilibrium is reached, 0.645 mol of CO₂ is in the container. Find the value of Keq for the reaction.arrow_forwardTrue or false. Why?arrow_forwardSuppose you have 1.25 moles of gaseous napthalene (C10H8) at T = 292 K. What is the average kinetic energy of one molecule of napthalene? (The molar mass of napthalene is 128 g/mol. You may assume that the gas molecules behave ideally.)arrow_forward
- A student experimentally determines the gas law constant, R, by reacting a small piece of magnesium with excess hydrochloric acid and then collecting the hydrogen gas over water in a eudiometer. Based L-atm on experimentally collected data, the student calculates R to equal 0.0832 mol·K L-atm Ideal gas law constant from literature: 0.08206 mol·K (a) Determine the percent error for the student's R-value. Percent error = % (b) For the statements below, identify the possible source(s) of error for this student's trial. The student notices a large air bubble in the eudiometer after collecting the hydrogen gas, but does not dislodge it. The student does not clean the zinc metal with sand paper. The student does not equilibrate the water levels within the eudiometer and the beaker at the end of the reaction. The water level in the eudiometer is 1-inch above the water level in the beaker. The student uses the barometric pressure for the lab to calculate R.arrow_forwardcontains 0.550 bar H2(g)0.550 bar H2(g) , 0.491 bar N2(g)0.491 bar N2(g) , and 0.121 bar Ar(g)0.121 bar Ar(g) . Calculate the mole fraction, ?χ , of each of these gases.arrow_forward7. When detern ing the mass of a liquid, ras in a hurry and did not allow enough time for the temperature of the flask to reach the temperature of the boiling water bath. The student recorded the temperature of the gas as 100.0°C when it was only 80.0°C, Is the molar mass calculated by the student higher or lower than the accepted molar mass, or does this error have no effect on the calculated result? Briefly explain your answer # 2/HB Office DEPOTS b) If the student assumed the volume of the flask was 125 mL (as marked by the side of the flask) rather than measuring the volume experimentally, would the unknown's calculated molar mass be too high, too low, or unaffected? Explain. # 2/HB 4arrow_forward
- 17 The total pressure is distinct from the individual partial pressures of a mixture. O True O False 身arrow_forwardWhat is kinetic energy of one mole of an ideal gas st 25C?arrow_forwardA gaseous mixture consists of 1.54 mol of helium, 0.36 mol of neon, and 1.58 mol of argon. What is the mole fraction of helium in the mixture?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY