
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
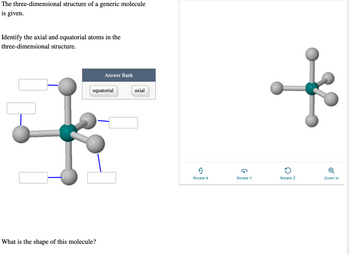
Transcribed Image Text:The three-dimensional structure of a generic molecule is given.
Identify the axial and equatorial atoms in the three-dimensional structure.
The diagram displayed is a three-dimensional molecular structure resembling a trigonal bipyramidal shape. It has a central atom shown in teal, with five surrounding atoms in gray connected via lines representing chemical bonds.
- The molecule has two distinct positions: three equatorial (in the same plane and forming an equilateral triangle around the central atom) and two axial (perpendicular to the equatorial plane).
- There is a labeled "Answer Bank" with the options "equatorial" and "axial" to fill the blank label boxes adjacent to each atom.
What is the shape of this molecule?
Options for interaction are provided at the bottom for rotating the structure along the X, Y, and Z axes, and for zooming in.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The chemical analysis of a water indicates the presence of cations in the following concentrations: Na+ 53 mg/L Mg2+36 mg/L K+ 72 mg/L Fe2+ 98 mg/L Mn2+15 mg/L A local softening company advertises that its softening unit has a capacity of 1000 meq. If water is used at the rate of 15 m³ per day, how frequently (i.e. how many times) will the unit have to be regenerated to provide the householder with soft water? (Na = 23, Mg = 24.4, K = 39, Fe = 55.85, Mn = 54.94 gms/mole) Answer: 105 Checkarrow_forwardConnectivity of the atoms of letter “B”naming the central atom and those atoms bonded to it. pointing out and stating the value of the bond angles in degrees; naming the geometrical shape of the molecule surrounding the central atom; discussing the polarity of the molecule.arrow_forwardWhat is the key conclusion we arrive at by analyzing the electrostatic forces involved in bonding?arrow_forward
- Determine the following for the molecule Ozone (O3) type of bonds: shape: polarity: bond angle:arrow_forwardWhat shape are molecules that have three total atoms and 20 total valence electrons?arrow_forwardExplain what is wrong with each molecular geometry and provide the correct molecular geometry, given the numbers of lone pairs and bonding groups on the central atom. Match the words in the left column to the appropriate blanks in the sentences on the right. a bent a trigonal bipyramidal an octahedral a trigonal planar a square planar a trigonal pyramidal a tetrahedral a seesaw a linear In structure (a), four pairs of electrons give cause lone pair-bonded pair repulsions and would have Reset electron geometry. The lone pair would molecular geometry. Help In structure (b), five pairs of electrons give electron geometry. The lone pair occupies an equatorial position to minimize lone pair-bonded pair repulsions, and the molecule would have molecular geometry. In structure (c), six pairs of electrons give electron geometry. The two lone pairs would occupy opposite positions to minimize lone pair-lone pair repulsions, and the molecule would have molecular geometry.arrow_forward
- What is the local molecular shape of the atom labeled "1?" What is the local molecular shape of the atom labeled "2?" What is the approximate value of the bond angle labeled "3?" What is the approximate value of the bond angle labeled "4?"arrow_forwardThe number of electron domains found around the central atom is known as the... electron number bond number valence number atomic number steric numberarrow_forwardOn scratch paper, draw the Lewis structure for CCl4 then, referring to the structure, fill in the blanks: The electron-pair geometry is The molecular geometry is The bond angle is The molecular polarity is (polar or non-polar?)arrow_forward
- Following is a molecule with polar bonds whose shape was obtained using the VSEPR theory. Specify the molecular shape of this molecule, and whether the molecule is polar or nonpolar. (Hint: In terms of polarity, see whether the dipoles in the molecule cancel or not. A molecule containing polar bonds can be nanpolar if the dipoles cancel each other. You can imagine the dipoles as ropes pulling on the central atom–If the pulls cancel each other, that is, the central atom cannot move, then the molecule is nonpolar. If on the other hand the opposite is true, then the molecule is polar.) O trigonal pyramidal shape, nonpolar O trigonal planar shape, nonpolar O tetrahedral shape, polar O trigonal pyramidal shape, polar O trigonal planar shape, polararrow_forwardWhat is the mole ratio of calcium carbonate to carbon dioxide in the reaction between hydrochloric acid and calcium carbonate? 2HCl + CaCO3 --> CaCl2 + CO2 + H2Oarrow_forwardIs carbon trichloride a polar molecule?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY