
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Can some help me, work through the problem in section (e) using my answers in section (a) trough (d)? I think the wording in section (e) has me a bit confused.
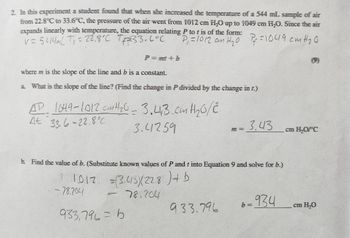
Transcribed Image Text:2. In this experiment a student found that when she increased the temperature of a 544 mL sample of air
from 22.8°C to 33.6°C, the pressure of the air went from 1012 cm H₂O up to 1049 cm H₂O. Since the air
expands linearly with temperature, the equation relating P to t is of the form:
V=5414mL T₁ = 22.8°C 7+2=33.6°C
P₁ =1012 an H₂0 Pf=1049 cm H ₂0
P=mmit b
where m is the slope of the line and b is a constant.
a. What is the slope of the line? (Find the change in P divided by the change in t.)
AD 1049-1012 cm H ₂0 = 3.41,3 cm H ₂0/2
At 33.6-22.8
3.41259
b. Find the value of b. (Substitute known values of P and t into Equation 9 and solve for b.)
1012
= (3.415)(228) + b
78.204
-78.204
=
933,796=6
7 = 3.43
933.796
-934
b=
cm H₂O/°C
cm H₂O

Transcribed Image Text:c. Express Equation 9 in terms of the values of mandi b.
d. At what temperature t will P become zero?
0=(3.43) 1934
9301
-9341= 13.436
P= (3.43) € + 934
-273
P = 0 at t =
°C = t₁ = -k
3.43
(Please note that you are unlikely to get results anywhere mear this good when actually carrying outt
this experiment, as it involves a very large extrapolation.)
e. The temperature in Part (d) is the absolute zero of temperature. Lord Kelvin suggested that we set up
a scale on which that temperature is OK. On that scale, T=t+ k. Show that, on the Kelvin scale, your
equation reduces to P= mil.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- On the molecules on the back page, circle and label all of the functional groups that you see (identified in the table above). Please note the following tips that may help you on this assignment: If a molecule contains –COOH, or a C double bonded to an O and single-bonded to an –OH, the functional group you circle is only the carboxyl, not a carboxyl, carbonyl, and hydroxyl separately). Phosphate groups are tricky – any P surrounded by 4 O’s is a phosphate – adjacent phosphates may share an O, and sometimes the O is in the form of OH. There is often shorthand that is used in drawing these structures – if you see a jagged line or ring structure, assume that carbon is at each corner. There may be ionized forms of the functional groups above (such as NH2 or NH3+) – these are the same functional groups. The order of the atoms in the functional group may be switched, depending on which side of the molecule it is on. For example, “—NH3” and “H3N—“ are the same thing.arrow_forwardWhich compound is acidic(increasing order)arrow_forwardFor the following reaction, identify the Brønsted-Lowry acid, base, the conjugate acid and base. Draw the correct curved arrow mechanism. predict the position of the equilibrium and identify the most acidic compound (stronger acid) and most basic compound (stronger base) || OH ||| . + IVarrow_forward
- In the following acid - base reactions, a) Draw Lewis structures of the reactants and the products. b) Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). c) Use the curved - arrow formalism to show the movement of electron pairs in these reactions and the imaginary movement in the resonance hybrids of the products. d) Indicate which reactions are best termed Brønsted-Lowry acid - base reactions i. CH3CHO + HCI-- > CH3CH2O + + Cl- ii. CH3CHO + OH- - - > CH3CO-(OH) Harrow_forward5. In each pair, which species is a stronger base? Justify your answer. Farrow_forwardis there another name for the first reactant in part c)?arrow_forward
- 3. (a) Draw the appropriate curved arrows and products for each set of reactants undergoing a coordination step. Identify each reactant species as either a Lewis acid or a Lewis base. (b) Use curved arrow notation to show each product undergoing heterolysis to regenerate reactants. (0) (ii) NH₂ H₂O + BF3 + AICI3arrow_forwardCircle the side that favored at equilibrium for the acid base reactions.arrow_forwardDraw a picture of a proton transfer reaction including curved arrowsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY