
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
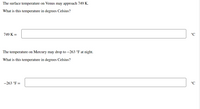
Transcribed Image Text:The surface temperature on Venus may approach 749 K.
What is this temperature in degrees Celsius?
749 K =
°C
The temperature on Mercury may drop to –263 °F at night.
What is this temperature in degrees Celsius?
-263 °F =
°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The boiling point of a liquid is calculated to be 377.44 K. What is this temperature on the Celsius and Fahrenheit scales? °C °Farrow_forwardWhile traveling in Europe, you see a weather forecast of 20.0°C. What is this temperature in °Farrow_forwardA Part A Planet X rotates in the same manner as the earth, around an axis through its north and south poles, and is perfectly spherical. An astronaut who weighs 943.0 N on the earth weighs 910.0 N at the north pole of Planet X and only 850.0 N at its equator. The distance from the north pole to the equator is 18,850 km, measured along the surface of Planet X. How long is the day on Planet X? Express your answer in hours. ΜΕ ΑΣΦ t = Submit Request Answer Part B ? h If a 45,000 kg satellite is placed in a circular orbit 4000 km above the surface of Planet X, what will be its orbital period? Express your answer in seconds. VAZO ΜΕ ΑΣΦ ? T = Submit Request Answer Sarrow_forward
- My lab partner writes the following calculation in our lab notebook: ((43.3 degrees C) (1.8 degrees F/1 degree C))+ (32 degrees F) = 109.9 degrees F What, if anything, is wrong with this calculation? Nothing; the calculation is fine as is When converting from degrees C to degrees F, you must add 32 degrees Fahrenheit before multiplying by the conversion ratio Only the conversion ratio relating degrees F to degrees C needs to be used here You need to subtract 32 degrees Fahrenheit, rather than adding them The calculation was done incorrectly, and this should really be 116. 2 degrees F Clear my selectionarrow_forwardThe wavelength for the color indigo is 445 nanometers (nm). What is this wavelength in kilometers (km)?arrow_forwardthe ionic radius of a sodium ion is 2.27 angstroms. what is this length in mmarrow_forward
- Washington, D.C. has an average January temperature of 34.9 degrees Fahrenheit. What is this average if temp is expressed in Celsius? (Round to two decimal places.) [Hint: Conversion between Fahrenheit and Celsius is a linear transformation: Fahrenheit = Celsius*1.80 + 32, or Celsius = Fahrenheit*(1/1.80) - (32/1.80)]arrow_forwardWhat temperature (in Kelvin) is a temperature of 51.67°C?arrow_forwardIn 1999, scientists discovered a new class of black holes with masses 100 to 10,000 times the mass of our sun, but occupying less space than our moon. Suppose that one of these black holes has a mass of 8×103 suns and a radius equal to one-half the radius of our moon. What is its density in grams per cubic centimeter? The mass of the sun is 2.0×1030kg and the radius of the moon is 2.16×103mi. (Volume of a sphere =43πr3.)arrow_forward
- In 1999, scientists discovered a new class of black holes with masses 100 to 10,000 times the mass of our sun, but occupying less space than our moon. Suppose that one of these black holes has a mass of 1×10^3 suns and a radius equal to one-half the radius of our moon. What is its density in grams per cubic centimeter? The mass of the sun is 2.0×10^30 kg and the radius of the moon is 2.16×10^3 mi (Volume of a sphere =4/3πr^3)arrow_forwarda swimming pool contains approximately 52.0 mL of water. What is the volume in cubic meters 1ml=1cm^3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY