Question
Transcribed Image Text:The specific heat capacity of steel is 450 J/kg-C
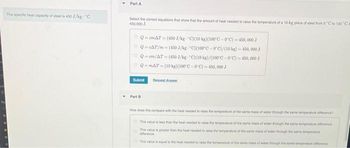
Part A
Select the correct equations that show that the amount of heal needed to raise the temperature of a 10-kg piece of steel from 0 C to 100 "Ca
450,000 J.
QemAT (450 J/kg "C)(10 kg)(100°C-0C)-450,000 J
ⒸQ-CAT/m-(450J/kg C)(100°C-0°C)/(10 kg) - 450,000 J
Q-cm/AT (450 J/kg-"C)(10 kg)/(100°C-0°C)-450,000 J
Q-mAT (10 kg)(100°C-0°C)-450,000 J
Submit
Part B
Request Answer
How does this compare with the heat needed to raise the temperature of the same mess of water through the same temperaturence?
This value is less than the heat needed to raise the temperature of the same mass of water through the same temperature offrance
This value is greater than the heat needed to raise the temperature of the same mass of water evough the same temperatur
difference
This value is equal to the heat needed to raise the temperature of the same mass of water through the same temperature fr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Change the following Fahrenheit temperature to Celsius temperature а. 80 ° F d. 150 ° F b. 15 ° F e. - 110 ° E C. - 14 ° Farrow_forwardQUESTION 19 A 0.03 kg lead bullet traveling 99 m/s strikes an armor plate and comes to stop. If all of the bullet'senergy is converted to heat that it alone absorbs, what is its temperature change? Specific Heat Capacity of the lead is 130//kg C g-9.8 m/s? Calculate to one decimal. QUESTION 20 Save Click Save and Submit fo save and submit. Click Save All Answers to save all ansu'ers. 85°F S 远arrow_forwardI need help with this questionarrow_forward
- Calculate the amount of heat energy transferred when 27.0 g of silver is heated from 50.0°C to 60.0°C. The specific heat capacity of silver is 0.0561 cal/g°C. A. 1.51 cal B. 15.1 cal C. -1.51 cal D. -15.1 calarrow_forward9. A glass rod is 0.50 m long and has a mass of 0.50 kg. When the rod is heated, its length is increased by 2.0 x 104 m. Calculate the amount of energy that is added to the rod as heat. Note: Cglass=840 J/(kg °C) and glass = 9.0 x 10-6 °C -¹.arrow_forward
arrow_back_ios
arrow_forward_ios