
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
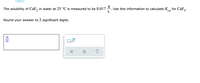
Transcribed Image Text:The solubility of CaF, in water at 25 °C is measured to be 0.017
Use this information to calculate Kn for CaF,.
L
sp
Round your answer to 2 significant digits.
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the solubility of Agl in a solution containing 0.100 M KI. Ksp Agl = 8.51x10-17. Enter the result in scientific notation to 2 decimal points. e.g enter 9.87x10-| as 9.87E-11.arrow_forwardCalculate the solubility of PbF, in water at 25 °C. You'll find K data in the ALEKS Data tab. sp Round your answer to 2 significant digits. g x10 ?arrow_forwardHOC3- = 4.8x10^-11arrow_forward
- based on the above information answer this plsarrow_forwardSoliud sodium phosphate is slowly added to a 75.0 mL of a 0.0586M calcium nitrate solution. The concentration of phosphate ion required to just initiate precipitation is M.arrow_forwardتھے Calculate the molar solubility calcium fluoride, CaF₂, given that Its Ksp 15 410 x 10" B. calculate molar solubility of CaF₂ in a 0,45M SOLUTION OF KF (aq) (include ICE chart)arrow_forward
- Complete the following solubility constant expression for Co (OH),. K - U sp =arrow_forwardPlease solve it correctly... posting third time and getting wrong answersarrow_forwardCalculate the solubility of BaCro, in water at 25 °C. You'll find K,, data in the ALEKS Data tab. Round your answer to 2 significant digits.arrow_forward
- solution. Calculate the solubility at 25 °C of Zn (OH), in pure water and in a 0.0030M ZNSO Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0030 M ZnSO, solution:arrow_forwardNitesharrow_forwardCalculate the solubility at 25 °C of BaCrO, in pure water and in a 0.0080M BaCl, solution. You'll find K, data in the ALEKS Data tab. sp Round both of your answers to 2 significant digits. g solubility in pure water: L g Ox10 solubility in 0.0080 M BaCl, solution:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY