
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
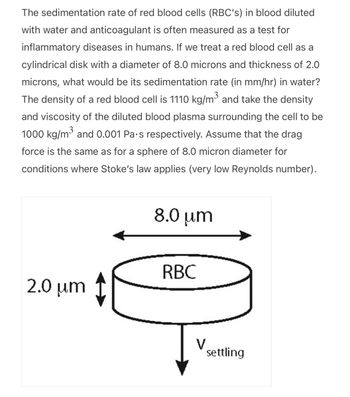
Transcribed Image Text:The sedimentation rate of red blood cells (RBC's) in blood diluted
with water and anticoagulant is often measured as a test for
inflammatory diseases in humans. If we treat a red blood cell as a
cylindrical disk with a diameter of 8.0 microns and thickness of 2.0
microns, what would be its sedimentation rate (in mm/hr) in water?
The density of a red blood cell is 1110 kg/m³ and take the density
and viscosity of the diluted blood plasma surrounding the cell to be
1000 kg/m³ and 0.001 Pa.s respectively. Assume that the drag
force is the same as for a sphere of 8.0 micron diameter for
conditions where Stoke's law applies (very low Reynolds number).
2.0 μm
8.0 μm
RBC
V
settling
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The concentration of cl-ion in a sample of H2o is ppm what mass of cl-ion is present in 240.0ml of H2o which has density of 1.00 g/mlarrow_forward1.6 Properties: Diffusivity Estimate the molecular diameter and diffusion coefficient for the proteins ribonuclease (MW 13,700 Da), hemoglobin (MW 68,000), and urease (MW 480,000), assuming the molecules are spherical and the density of each protein molecule is 1.3 g/cm³.arrow_forward1arrow_forward
- A reverse-osmosis unit is used to obtain pure water from saline water. The coefficient of water permeation is 0.1 mol/cm2 s atm. The pressure differential across the membrane is 0.2 atm, and the osmotic pressure differential is 0.05 atm. The water flux across the membrane is most nearly (A) 0.0050 mol/cm2·s (B) 0.015 mol/cm2·s (C) 0.050 mol/cm2·s (D) 0.15 mol/cm² ·sarrow_forwardAcetic acid (CH3COOH) diffuses across a 1-mm thick film of non-diffusing water. The concentration of acetic acid on opposite sides of water film are 9% by weight (density=1012 kg/m³) and 4% by weight (density=1003.2 kg/m³). Calculate the rate of diffusion of acetic acid at 17 °C. DATA: The diffusivity of acetic acid in water at 25 °C = 1.11 x 109 m²/s Viscosity of acetic acid solution at 25 °C = 1.1336 x 10-³ Pa. Viscosity of acetic acid solution at 17 °C = 1.2883 x 10-³ Pa.sarrow_forwardThe diffusion constant for the amino acid glycine in water is 1.06 × 10-9 m2/s. In a 2.0-cm-long tube with a cross-sectional area of 1.3 × 10-4m2, the mass rate of diffusion is m/t = 4.2 × 10-14 kg/s, because the glycine concentration is maintained at a value of 9.1 × 10-3 kg/m3 at one end of the tube and at a lower value at the other end. What is the lower concentration?arrow_forward
- A// Fick's first diffusion law states that: Mass flux = -D Where mass flux = the quantity of mass (g/cm²/s), D a diffusion coefficient (1.6×10-6 cm²/s), c concentration, and x = distance (cm). An environmental engineer measures the following concentration of a pollutant in the sediments underlying a lake as the following: 0 2 6 0.12×10-6 0.64x10-6 1.20×10-6 Use a suitable Newton's interpolation formula available to estimate the derivative at x = 0 and then calculate the mass flux of pollutant. X, cm C, g/cm³ de dx =arrow_forwardA quiescent body of water has a depth of 600 mm. The DO level at the bottom after 16 days is 3.5 mg/l when the surface is exposed to the atmosphere at a temperature of 25o Determine the DO of the water body. The saturated DO at 25oC is 8.5 mg/l and the diffusion coefficient (kd) at 25oC is 2.5 x 10-3 mm2/s.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The