
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
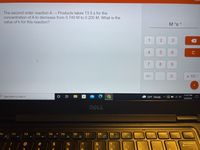
Transcribed Image Text:The second order reaction A → Products takes 13.5 s for the
concentration of A to decrease from 0.740 M to 0.205 M. What is the
value of k for this reaction?
M 's 1
3.
9.
C
7
8.
9.
+/-
x 100
11:47 PM
OType here to search
53°F Cloudy ^ E O
5/8/2022
DELL
Esc
F1
F2
F3
F4
F5
F6
F7
F8
F9
F10
F11
F12
PrtScr
Insert
Delete
Fn
!
@
#
2$
&
Backspace
08.
1
3
2.
Co
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the concentration of A after 15.5 minutes for the reaction A → Products when the initial concentration of A is 0.750 M? (k = 0.0451 M-¹min-¹)arrow_forwardThe second order reaction A→ Products takes 13.5 s for the concentration of A to decrease from 0.740 M to 0.265 M. What is the value of k for this reaction?arrow_forwardReaction rates can only be determined by studying the change in concentration of the products. Question 3 options: True Falsearrow_forward
- The second order reaction A → Products takes 13.5 s for the concentration of A to decrease from 0.740 M to 0.213 M. What is the value of k for this reaction?arrow_forwardk=? k=?arrow_forwardWhat is the concentration of A after 37.5 minutes for the reaction A → Products when the initial concentration of A is 0.750 M? (k = 0.0451 M⁻¹min⁻¹)arrow_forward
- How do I calculate k when [X]= .42 M and the rate of reaction is .0030 M/s. I can't remember exactly how to do this type of problem. Thank you for the help !arrow_forwardConsider the quilibrium reaction between X and Y, as shown below: X=Y AG The reaction is started with 10 mmol of X; no Y is initially present. After 48 hours, analysis reveals the presence of 10 mmol of X and 0 mmol of Y. Which is the most likely explanation? = −1 - 45 kJ mol X and Y have reached equilibrium concentrations. An enzyme has shifted the equilibrium toward X. Formation of Y is kinetically slow; equilibrium has not been reached by 48 hours. Formation of Y is thermodynamically unfavorable. Two of the above explanations are reasonable.arrow_forwardWhat is the concentration of A after 30.5 minutes for the reaction A → Products when the initial concentration of A is 0.750 M? (k = 0.0451 M⁻¹min⁻¹)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY