
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
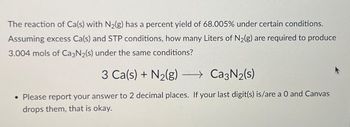
Transcribed Image Text:The reaction of Ca(s) with N₂(g) has a percent yield of 68.005% under certain conditions.
Assuming excess Ca(s) and STP conditions, how many Liters of N₂(g) are required to produce
3.004 mols of Ca3N₂(s) under the same conditions?
3 Ca(s) + N₂(g) →→→ Ca3N₂(s)
• Please report your answer to 2 decimal places. If your last digit(s) is/are a 0 and Canvas
drops them, that is okay.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to the following reaction:2C4H10 (g) + 13O2 (g) 8CO2 (g) + 10H2O (g)arrow_forwardThe Apollo 13 mission astronauts are running out of oxygen and need to get rid of the excess carbon dioxide. You know that sodium hydroxide can remove carbon dioxide from the spacecraft cabin. The filter which they had been using is fully saturated and no longer works. The astronauts have a 5 kg container of sodium hydroxide on the ship. The reaction to neutralize the air, remove carbon dioxide with sodium hydroxide is shown below CO2(g) + 2NaOH(s) ⟶⟶ Na2CO3(aq) + H2O(l) The astronauts have 2 days left before they land on earth. You know that there are three astronauts, and each astronaut emits roughly 500. g of carbon dioxide each day. Is there enough sodium hydroxide in the cabin to cleanse the cabin air of the carbon dioxide, or are the astronauts doomed? Calculate how much sodium hydroxide is required to remove all the carbon dioxide from the three astronauts. Be sure to show all your work!arrow_forwardBalance this unbalanced equation by entering only whole numbers into the blank. If the molar coefficient is 1, enter "1" (do not leave it blank: CaHg + O2 → CO2 + H20arrow_forward
- You have 22.2 g of the compound C2Hx, where x is the maximum number of hydrogens that can fit around two carbon atoms and still create a stable Lewis dot structure. If you allow all of this sample to react with excess oxygen and then allow the products to cool to room temperature, how many moles of liquid product will you have?arrow_forwardConsider the reaction below LiOH+KCL LiCL+KOH If 20.0 grams of lithium hydroxide LiOH are used, what is the theoretical yield of lithium chloride LiCL?arrow_forwardIf you wanted to produce 4 moles of SO3 gas, how many moles of SO2 would you need to react? (Assume an unlimited supply of O2 gas.) 2 SO₂ (g) + O₂(g) →2 SO, (g)arrow_forward
- A studen wanted to determine the value of R and so used the reaction: Mg(s) + 2 HCl(aq) → H2(g) + MgCl2 (aq) She got a piece of magnesium ribbon and determined its mass as 0.0841 g. She placed the ribbon in a test tube with water and excess HCl and inverted it to allow the H2 gas to bubble to the top of the tube while water was pushed out the bottom. She measured the volume of the gas in the tube as 87.8 mL. She measured the temperature of the water at 20.5ᵒC and the atmospheric pressure in the room was 751 torr. 1- What other piece of information did she have to determine in order to accurately calculate R? 2- What did she calculate for the value of R? 3- What was her % error?arrow_forwardSuppose a 250. mL flask is filled with 1.0 mol of CO, 0.70 mol of H₂O and 2.0 mol of H₂. This reaction becomes possible: CO(g) + H₂O(g) CO₂(g) + H₂(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CO₂. You can leave out the M symbol for molarity. initial change equilibrium 2 со 0 H₂O CO₂ X H₂ 0 X 5arrow_forward15. Citric acid, C6H807 (aq), is often used as an additive to make candies "sour. a. Draw a flowchart that shows how to caloulate the molar mass of citric acid, b. Verify the steps in your flowchart by calculating the molar mass of citric acid.arrow_forward
- A 6.79 g sample of NH, gas and a 6.79 g sample of HCl gas are mixed in a 1.50 L flask at 25 °C. < EFeedback You have not correctly determined the mass of NH,Cl formed. Identify the limiting reagent. Convert the given mass of the limiting reagent to the mass of NH,Cl using the NH; mole ratio from the balanced chemical HCI equation and the molar masses for both NH,CI the limiting reagent and the NH,Cl as conversion factors. How many grams of NH,Cl will be formed by this reaction? 0.273 mass: Incorrect What is the pressure in atmospheres of the gas remaining in the flask? Ignore the volume of solid NH,Cl produced by the reaction. P = 3.42arrow_forwardCalcium hydride reacts with water to produce hydrogen gas according to the following reaction: CaH2(s) + 2 H2O(l) ----> 2H2(g) + Ca(OH)2(aq) This reaction is used to generate hydrogen gas to inflate air bags in cars and life rafts on boats and for similar uses where a simple compact means of hydrogen generation is necessary. Assuming complete reaction with water, how many grams of calcium hydride are required to fill a raft to a total pressure of 2.0 atm at 25oC if the volume of the raft is 1,000 L?arrow_forwardBefore the use of CFCs, sulfur dioxide was used as a refrigerant. A sample of sulfur dioxide undergoes a simple decomposition reaction as follows: 8 SO2(g) --> S8(s) + 8 O2(g) Calculate the number of moles of oxygen gas produced in addition to the production of 1.18 mol of sulfur.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY