
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
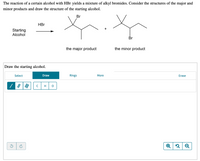
Transcribed Image Text:The reaction of a certain alcohol with HBr yields a mixture of alkyl bromides. Consider the structures of the major and minor products and draw the structure of the starting alcohol.
**Diagram Description:**
1. **Reaction Scheme:**
- The starting alcohol reacts with HBr.
- Two products are formed:
- The major product, shown with a bromine (Br) atom attached to a secondary carbon in the molecule.
- The minor product, shown with a bromine (Br) atom attached to a primary carbon in the molecule.
2. **Product Structures:**
- **Major Product:** The structure on the left has the bromine atom attached to a more substituted tertiary carbon.
- **Minor Product:** The structure on the right has the bromine atom attached to a less substituted primary carbon.
**Drawing Section:**
- Below the reaction, there is a drawing interface labeled "Draw the starting alcohol."
**Tools Provided:**
- Options to select, draw, use pre-defined rings, and access more drawing tools.
- Element buttons for carbon (C), hydrogen (H), and oxygen (O).
- A blank canvas to draw on with undo and redo buttons.
- Additional viewer tools: zoom in, zoom out, and reset view.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the following structure undergoes oxidation reaction, which of the product(s) will form: ОН H2C–CH2-CH2 CH3 O A. Aldehydes only O B. This molecule cannot go through oxidation reaction. OC. Ketone O D. Aldehyde & Carboxylic acid O E. Ether and waterarrow_forwardWhich of the following is a tertiary alcohol? HC CH H.C HC OI O III OIV CH HO H₂C H₂C H₁C H.C CH H₂ CH CH CH H.C CH "CH, COH IV H.C CH OH H,C CH₂ CH, OHarrow_forwarda primary alcohol has one -OH group, a secondary hasarrow_forward
- What is the classification of the following structure? OH H2C-CH2 CH2 CH3 O A. Primary Alcohol OB Secondary Alcohol OC. Tertiary Alcohol OD. Phenolarrow_forward1. When an aldehyde is reduced with hydrogen (H2) gas in the presence of a transition metal catalyst, what type of product is formed? A. primary alcohol B. secondary alcohol C. tertiary alcohol D. carboxylic acidarrow_forwardDescribe an experimental test that you would perform to distinguish between a primary alcohol, an alkene and an aldehydearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY