
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
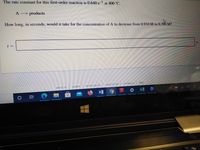
Transcribed Image Text:The rate constant for this first-order reaction is 0.640 s at 400 °C.
A products
How long, in seconds, would it take for the concentration of A to decrease from 0.910 M to 0.390 M?
terms of use
contact us help
about us
careers
privacy policy
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the half life, in seconds, for a first order reaction with an initial concentration of 0.104 M and a rate constant, k, of 0.148 1/s.arrow_forwardThe reactant concentration in a second-order reaction was 0.230 MM after 230 s and 4.20×10−2 MM after 725 s . What is the rate constant for this reaction?arrow_forwardThe decomposition of sulfuryl chloride, SO2Cl2, to sulfur dioxide and chlorine gases is a first-order reaction. It is found that at a certain temperature, the rate constant equals 9.19 × 10-6 s-1. How long will it take to decompose SO2Cl2 so that 30.0% remains? The starting concentration is 0.0217 M.arrow_forward
- The rate constant for this first‑order reaction is 0.910 s−1 at 400 ∘C. A⟶products How long, in seconds, would it take for the concentration of A to decrease from 0.830 M to 0.310 M?arrow_forwardThe rate constant for a first order reaction is 0.54 s-1. If a 1.57 M sample reacts for 12 seconds, what concentration (in molarity) will remain?arrow_forwardNitesharrow_forward
- The rate constant of a first-order reaction is 4.60 x 10* s at 350 degrees Celsius. If the activation energy is 104 kJ/mol, calculate the temperature at which its rate constantis 8.80 x 104 s?.arrow_forwardThe rate constant for this first-order reaction is 0.0125 s−10.0125 s−1 at 300 °C. A⟶productsA⟶products If the initial mass of A is 18.99 g,18.99 g, calculate the mass of A remaining after 2.53 min.arrow_forwardThe rate constant for the reaction2A → Bis 7.55 × 10−3 s−1 at 110°C. The reaction is first order in A. How long (in seconds) will it take for [A] to decrease from 1.27 M to 0.510 M?arrow_forward
- The rate constant for this second-order reaction is 0.780 M-1 ·s-1 at 300 °C. A products How long, in seconds, would it take for the concentration of A to decrease from 0.800 M to 0.390 M?arrow_forwardThe rate constant for this zero-order reaction is 0.0170 M ·s at 300 °C. A → products How long (in seconds) would it take for the concentration of A to decrease from 0.860 M to 0.320 M? t = Sarrow_forwardThe rate constant for this first-order reaction is 0.720 s -1 at 400 °C. A → products How long, in seconds, would it take for the concentration of A to decrease from 0.890 M to 0.330 M? t = Sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY