
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
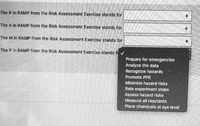
Transcribed Image Text:**Understanding the RAMP Process in Risk Assessment**
The RAMP process is crucial for identifying and mitigating hazards in any risk assessment exercise. Each letter in "RAMP" stands for a specific step in the process, which ensures comprehensive evaluation and management of potential risks. This section will help you understand what each letter represents and how it contributes to the overall risk management strategy.
**RAMP Breakdown:**
- **The R in RAMP from the Risk Assessment Exercise stands for:**
- Recognize hazards
- **The A in RAMP from the Risk Assessment Exercise stands for:**
- Assess hazard risks
- **The M in RAMP from the Risk Assessment Exercise stands for:**
- Minimize hazard risks
- **The P in RAMP from the Risk Assessment Exercise stands for:**
- Prepare for emergencies
**Options Available:**
When determining what each letter stands for, you may encounter several options:
- Prepare for emergencies
- Analyze the data
- Recognize hazards
- Promote PPE (Personal Protective Equipment)
- Minimize hazard risks
- Rate experiment steps
- Assess hazard risks
- Measure all reactants
- Place chemicals at eye level
By understanding these components, you can effectively conduct a risk assessment that identifies, evaluates, mitigates, and prepares for potential hazards, ensuring a safer working environment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Standard white vinegar you can buy in the grocery store is 5% concentration. That means 5% of the liquid vinegar is acetic acid and 95% of the solution is water. In a hardware store, you can buy industria strength vinegar, which is 30% concentration. This means that 30% of the vinegar is acetic acid, and the remaining 70% is water. Samuel does another experiment, this time with 5% vinegar and 30% vinegar. He sets up two science fair volcanoes (in no particular order), each with the same temperature, mass of baking soda and volume o vinegar. But one volcano uses 5% vinegar and the other volcano uses 30% vinegar. He measures the volume of gas production for the'first minute of each reaction, and he records the data below. Volcano # 1 Volume of gas produced (mL.) vs. Time (s) for Volcano #1 Time Volume of gas (s) produced (ml) 60 E 50 10 20 30 25 8 40 38 46 30 20 Volume of gas produced (ml) 40 50 50 60 52 of 10 53 20 40 60 80 Time (s) Volcano # 2 Volume of gas Volume of gas produced…arrow_forwardHelp pleasearrow_forwardHydrochloric acid can be prepared by the following reaction: 2NACI(s) + H2SO4(aq)2HCI(g) + Na2SO4(s) What mass of HCI can be prepared from 2.00 mol H SO4 and 150. g NaCl? Multiple Choice 2.57 g 167 g 93.6 g 11 asus COLLECTION prt sc delete 112/A insert f7 f8 f11arrow_forward
- -CHEM A054 520 2 X Question 10 - Chapter 9 part 2 X CH6_Chem103 - Kenai Peninsu x + ezto.mheducation.com/ext/map/index.html?_con=con&external_browser=0&launch Chapter 9 part 2 Homework i 10 1 points 8 01:46:48 eBook Hint Print References Mc Graw Hill 7 2 Determine the mass of Na2SO3 necessary to prepare 500.0 mL of a solution that is 0.168 M Na2SO3. g Na2SO3 3 80 00 F3 $ 4 000 000 F4 dº LO % 5 8 DII F8 ( 9 2. F9 (arrow_forwardPlease help me BALANCE this chemical equations (just exercises 19-27)arrow_forwardQuestion Complete and balance the equation for the reaction of hydrobromic acid (HBr) with strontium metal (Sr). • Write the chemical equation in molecular form (do not show dissociated ions). • Include the state (phase) of each chemical species. Provide your answer below: HBr(aq) + Sr(s) → FEEDBACK MORE INSTRUCTION SL Content attributionarrow_forward
- quiz 11 for chemistry lab - Compatibility Mode - Saved - nerbsl@yahoo.com Design Layout References Mailings Review View Help OFind S Replace A A Aav Ao AaBbCcDc AaBbCcDc AABBCC AABBCCC A- D. A - 三。 、、 1 Normal 1 No Spac. Heading 1 Heading 2 A Select Paragraph Styles Editing 6. If the Cu metal loses more mass than the other metal gains, the missing mass of Cu could a) be in the CuSOA(ag) solution b) have deposited somewhere else in the electroplating apparatus c) have radioactively decayed d) a) or b) 7. If the other metal gains more mass than the Cu metal loses, the extra mass of Cu could a) have come from the CuSO.(ag) solution b) have been created from electrical energy c) both of these d) none of thesearrow_forwardRefer to Figure 1 and answer the following questions: (i) (ii) Pictograms are symbols used in Global Harmonized System (GHS) to make hazard communication more effective. Use one key word to describe the meaning of Pictogram 1, 3, 4, 8 respectively. Provide an example of chemical compound for each. Assign relevant pictograms to the following activities. Activity 1: Heating sodium chloride with concentrated sulfuric acid Activity 2: Preparing aqueous sodium borohydride in a closed glass vessel Activity 3: Adding diethylene glycol to potassium permanganate solid 1 4 2 5 3 6 th 7 8 9 Figure 1. Pictograms under GHS system.arrow_forwardferences m Post-Lab Q... Mailings BLE 11 Post-Lab Questions: Review View Help 2. KT 3 ✔ 2 afusat.yinusa-W211098127 G ** 1. If a student did not sufficiently dry the sand or salt, what affect will it have on the overall mass recovered measured and mass percentage of the recovered mixture? Iarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY