
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
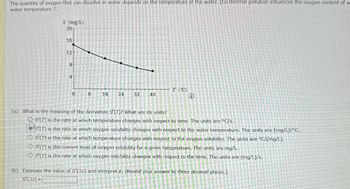
Transcribed Image Text:The quantity of oxygen that can dissolve in water depends on the temperature of the water. (So thermal pollution influences the oxygen content of w
water temperature T.
S (mg/L)
20
16
12
8
4
0
8
16 24
32
40
T (°C)
0
(a) What is the meaning of the derivative S'(7)? What are its units?
S'(T) is the rate at which temperature changes with respect to time. The units are °C/s.
S(T) is the rate at which oxygen solubility changes with respect to the water temperature. The units are (mg/L)/°C.
O S'(T) is the rate at which temperature changes with respect to the oxygen solubility. The units are °C/(mg/L).
OS'(T) is the current level of oxygen solubility for a given temperature. The units are mg/L.
OS'(T) is the rate at which oxygen solubility changes with respect to the time. The units are (mg/L)/s.
(b) Estimate the value of S'(32) and interpret it. (Round your answer to three decimal places.)
S(32)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- For a particular isomer of Cg H₁g, the combustion reaction produces 5108.7 kJ of heat per mole of C, H₁g (g) consumed, under standard conditions. 25 Cg H18 (8) + O₂(g) →→→ 8 CO₂(g) + 9 H₂O(g) What is the standard enthalpy of formation of this isomer of Cg H₁g (g)? AH; = $ V T B MacBook Air Y H & 7 8 AH-5108.7 kJ/mol 18 N M K 1 38 kJ/molarrow_forwardEry 1 3 M (M) 2req The production capacity for acrylonitrile (C3H3N) in the United States is over 2 billion pounds per year. Acrylonitrile, the building block for polyacrylonitrile fibers and a variety of plastics, is produced from gaseous propylene, ammonia, and oxygen: 2C3H6(g) + 2NH3(g) + 30₂(g) → 2C3H3N(g) + 6H₂O(g) Assuming 100% yield, determine the mass of acrylonitrile which can be produced from the mixture below: Mass 7.28x102 g 5.00 x 102 g 1.00 x 103 g In progress Reactant oxygen propylene ammonia oxygen What mass of water is formed from your mixture? mass of water formed = g Calculate the mass (in grams) of each reactant after the reaction is complete: Submit g = mass of acrylonitrile produced Reactant Mass remaining propylene ammonia Show Hints ס ס ס g g Cengage Learning Cengage Technical Support Previous Next Save and Exitarrow_forwardSome commercial drain cleaners use a mixture of sodium hydroxide and aluminum powder. When the solid mixture is poured into the drain and dissolves, a reaction ensues that produces hydrogen gas: 2N2OH(aq) + 2Al(s) + 6H2O() – 2NAAI(OH)4(aq) + 3H2(g) Determine the temperature (in °C) of hydrogen gas produced when 31.58 g of aluminum reacts with excess sodium hydroxide and water if the pressure is 103.10 kPa and the volume is 36.23 L. Provide your answer with TWO decimals. Your Answer: Answer unitsarrow_forward
- CaCO3(s)⇄CaO(s)+CO2(g) When heated strongly, solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas, as represented by the equation above. A 2.0mol sample of CaCO3(s) is placed in a rigid 100.L reaction vessel from which all the air has been evacuated. The vessel is heated to 898°C at which time the pressure of CO2(g) in the vessel is constant at 1.00atm, while some CaCO3(s) remains in the vessel. (a) Calculate the number of moles of CO2(g) present in the vessel at equilibrium. (b) Write the expression for Kp, the equilibrium constant for the reaction, and determine its value at 898°C. (c) The experiment was repeated, but this time starting with a 4.0mol sample of CaCO3(s). On the following graph, draw a curve showing how the pressure of CO2(g) would change over time as the vessel is heated to 898°C and equilibrium is established.arrow_forwardAn industrial process for manufacturing sulfuric acid, H2SO4, uses hydrogen sulfide, H2S, from the purification of natural gas. In the first step of this process, the hydrogen sulfide is burned to obtain sulfur dioxide, SO2. 2H2S(g) + 3 O2(g) → 2 H2O(l) + 2 SO2(g); ∆H = -1124 kJ. The density of sulfur dioxide at 25 °C and 1.00 atm is 2.62 g/L, and the molar heat capacity is 30.2 J/mol °C. a) How much heat would be evolved in producing 1.00 L of SO2 at 25 °C and 1.00 atm? b) Suppose heat from this reaction is used to heat 1.00 L of SO2 from 25 °C to 500 °C for its use in the next step of the process. What percentage of the heat evolved is required for this?arrow_forwardSome commercial drain cleaners use a mixture of sodium hydroxide and aluminum powder. When the solid mixture is poured into the drain and dissolves, a reaction ensues that produces hydrogen gas: 2NAOH(aq) + 2AI(s) + 6H2O() → 2NAAI(OH)4(aq) + 3H2{g) Determine the temperature (in C) of hydrogen gas produced when 17.08 g of aluminum reacts with excess sodium hydroxide and water if the pressure is 107.37 kPa and the volume is 40.29 L. Provide your answer with TWO decimals.arrow_forward
- Oxygen gas can be generated by heating potassium chlorate: heat 2KCIO, (s) 2KCI(s) + 30, (g) What volume of oxygen gas, collected by displacement of water and measured at 750.1 torr and 80.0 °C (vapor pressure of water 80.0 °C -355.63 torr), will be formed by the decomposition of 19.3 g of potassium chlorate? Be sure your answer has the correct number of significant figures. Note: Reference the Conversion factors for non-SI units and Fundamental constants tables for additional information. L D-P X Sarrow_forwardjust 2 and 3 and maybe 4arrow_forwardDATA : Mass of Magnesium (g) 0.0525 g Volume of hydrogen gas (mL) 50.0 mL Temperature of hydrogen gas (°C) 25.0°C Atmospheric Pressure (mmHg) 762 mm Hg Vapor pressure of water (mmHg) Partial pressure of hydrogen (mmHg) DATA ANALYSISarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY