
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
True or false.
if the statement is FALSE, place the correct word/s that should replace the word/s in capital letters to make the statement true.
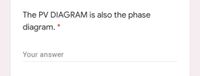
Transcribed Image Text:The PV DIAGRAM is also the phase
diagram.
Your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- I need help with molarity of NaOHarrow_forwardAutoSave Document1 - Word O Search (Alt+Q) ff RICAMAE SALADAGA RS File Home Insert Draw Design Layout References Mailings Review View Help P Comments B Share X Cut O Find v Calibri (Body) - A A Aa v A AаBЬСcDd AaBbСcDd AaBbC AаBЬСc[ Аа B АавьссD v 11 LE Copy Replace Paste BIUV ab x, x A . I v A - I Normal 1 No Spac. Heading 1 Heading 2 Title Subtitle Dictate Sensitivity Editor Reuse S Format Painter A Select v Files Clipboard Font Paragraph Styles Editing Voice Sensitivity Editor Reuse Files |· 2: 1 1 |· ·1: 3.1. 4 5.. 6 . I 7.1· 8.I 10.1 11.| 12:1 13. 14. I 15. 16 17.1 18 | 19 II. The adiabatic exothermic irreversible gas-phase reaction 2A + B → 20 is to be carried out in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown in the figure: 500,000 400,000 300,000 FAO -rA 200,000 100,000 0.3 0.6 0.9 1. What PFR volume is necessary to achieve 50% conversion? 2. What CSTR volume is necessary to achieve 50% conversion? 3. What is the volume of a…arrow_forwardWith the data given, prepare a plot of Absorbance vs. Wavelength. Choose options that: draw a smooth line through the data points, label the axes, and include an appropriate title.arrow_forward
- The elevation of the water table at well D is O 158.1 O 7.8 165.9 150.3 The general direction of groundwater flow is Onortheast O southeast O southwest O northwest Trichloroethylene (TCE) is a solvent that has commonly been used as a cleaning agent O a flavoring agent O an insecticide O a lubricant The owners of Chemical Plant A have filed a lawsuit against the owners of Chemical Plant B for polluting the groundwater beneath their site with trichloroehtylene (TCE). Based on the groundwater flow direction shown on your map, does Chemical Plant A have a valid case? O Yes feet. O No Their is a lack of information to make this determinationarrow_forwardEstimate the cut diameter and overall collection efficiency of a cyclone given the particle size distribution containing algae. Particle size distribution and other pertinent data are given below. Average particle size dp, μm 1 5 10 20 30 40 50 60 >60 Wt % 3 20 15 20 16 10 6 3 7 Other design parameters are: Gas viscosity = 0.02 Cp; Specific Gravity of the particle = 3.0; Inlet gas velocity of cyclone = 14.6 m/s Effective number of turns within cyclone = 5 Cyclone diameter = 2.44 m Cyclone inlet width = 0.61 marrow_forwardXi = уір ресто drive to get this equation lap = when УА + Ув YA Ус PA(T) PALT) PACT)arrow_forward
- A. yield stress B. ultimate tensile stress C. fracture stress D. elastic deformation region E. plastic deformation regionarrow_forwardAich's alloy, a brass alloy contains 60.66% copper, 36.58% zinc, 1.02% tin, and 1.74% iron. Jimmy John has a 250.1 kg block of this alloy in the machine shop. Determine the mass of copper (in kg), zinc (in lb), tin (in g), and iron (in mg) in this brass block. What are common areas of use for Aich's alloy?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The