
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The pure water flux of a membrane with a diameter of 7.5 cm has to be determined
as a function of the applied pressure (see Table). The following results are obtained. Determine the water permeability coefficient by means of a graph
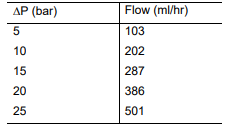
Transcribed Image Text:AP (bar)
5
10
15
20
25
Flow (ml/hr)
103
202
287
386
501
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Only typed solutionarrow_forward(a) Water supplies are often treated with chlorine as one of the processing steps in treating wastewater. Estimate the liquid diffusion coefficient of chlorine in an infinitely dilute solution of water at 289 K using the Wilke-Chang equation. (b) Estimate the liquid diffusivity of the following solutes that are transferred through dilute solutions: (i) oxygen in water at 18ºC; (ii) CO2 in water at 25ºC.arrow_forwardThe molar conductivity at infinite dilution of hydrobromic acid, sodium acetate, & sodium bromide at 298 K are 520.1 Scm2mol-1, 105.9 Scm2mol" 1, and 186.3 Scm?mol-1, respectively. Determine the molar conductivity at infinite dilution of acetic acid.arrow_forward
- The Debye radius in a solution with an ionic strength of 75.0 mol/m3 was determined to be 0.301 nm. a. If all factors remain constant, but an ionic strength of 15.0 mol/m3 is used instead of 75.0 mol/m3, calculate the Debye radius. b. Which condition is more favorable for particle-particle attachment?arrow_forwardAluminum atoms are to be diffused into a silicon wafer using both predeposition and drive-in heat treatments; the background concentration of Al in this silicon material is known to be 2.5 x 101⁹ atoms/m³. The drive-in diffusion treatment is to be carried out at 1050°C for a period of 4.0 h, which gives a junction depth x; of 3.0 μm. Compute the predeposition diffusion time at 950°C if the surface concentration is maintained at a constant level of 2 x 1025 atoms/m³. For the diffusion of Al in Si, values of Qd and Do are 3.41 eV and 1.38 x 10-4 m²/s, respectively. minarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY