
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
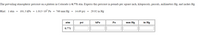
Transcribed Image Text:The prevailing atmospheric pressure on a plateau in Colorado is 0.771 atm. Express this pressure in pounds per square inch, kilopascals, pascals, millimeters Hg, and inches Hg.
Hint: 1 atm =
101.3 kPa =
1.013×10° Pa
= 760 mm Hg
14.69 psi = 29.92 in Hg
atm
psi
kPa
Ра
mm Hg
in Hg
0.771
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Search [Review Topics] [References] Use the References to access important values if needed for this question. A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 733 mm Hg. Express this pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals. Hint: 1 atm = 101.3 kPa = 760 torr = 760 mm Hg Submit Answer Retry Entire Group mm Hg 733 1 = 14.69 psi = 1.013×105 Pal atm kPa torr psi 9 more group attempts remaining Pa Previous Next Save andarrow_forwardA student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 714 mm Hg. Express this pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals. Hint: 1 atm = 101.3 kPa = 760 torr = 760 mm Hg = 14.69 psi = 1.013×105 Pa mm Hg 714 atm kPa torr psi Paarrow_forwardeq req < 1req For each of the following sets of volume/temperature data, calculate the missing quantity after the change is made. Assume that the pressure and the amount of gas remain constant. V=2.30 L at 22.0 °C V = 4.98 L at ? °C PC V = 120. mL at 281 K V = ? mL at 386 K mL V=49.7 mL at 37.0 °C V = ? mL at 357 K a. APR 30 b. C. Submit Answer 2 mL Retry Entire Group Show Hint tv [Review Topics] Ⓒ 9 more group attempts remaining [References] SENSA SPREC ********* Previous Next Save and Exit 3arrow_forward
- Which of the following equations shows an incorrect relationship between pressures given in terms of different units? A 0.760 atm = 578 mm Hg В 152 mm Hg = 2.03 × 104 Pa %3D 1.0 torr = 2.00 mm Hg D 1.00 atm = 760 torr (E 1.20 atm = 122 kPaarrow_forwardPam The pressure of a sample of gas is measured with an open- ended manometer, shown to the right. The atmospheric pressure is 758 mm Hg. The liquid in the manometer is mercury. What is the pressure of the gas in mm Hg? (1 cm 10 mm) 1. Gas Open end 5.13 cm The total pressure in a tire is 1.20 atm at 23.0 °C. If the temperature is increased to 47.0 °C, what is the new pressure in the 2. tire? Assume the volume of the tire does not change.arrow_forwardStudy the following phase diagram of Substance X. pressure (atm) 1.6- 0.8- solid 200 liquid temperature (K) 400 - gas Use this diagram to answer the following questions.arrow_forward
- An elemental gas has a mass of 10.3 g. If the volume is 58.4 L and the pressure is 0.997 atm at a temperature of 25.0 degrees celsius, what is the gas?arrow_forwardThe gas of a certain volcano had the following composition in mole percent: 60.0% CO2, 28.0% H2, 7.40% HCl, 1.80% HF, 2.70% SO2, and 0.100% H2S. What would be the partial pressure of HF in atmospheres if the total pressure was 285 mmHg? 1atm = 760mmHgarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY