
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
The pressure difference between two air tanks A and B is measured by a U - tube manometer with mercury as the manometer liquid. The barometric pressure is 700 mm Hg.
a. What is the absolute pressure in the tank A?
b. What is the gauge pressure in the tank A?
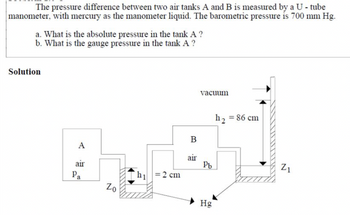
Transcribed Image Text:The pressure difference between two air tanks A and B is measured by a U-tube
manometer, with mercury as the manometer liquid. The barometric pressure is 700 mm Hg.
a. What is the absolute pressure in the tank A?
b. What is the gauge pressure in the tank A ?
Solution
A
air
Pa
Zo
h₁
= 2 cm
B
air
vacuum
Pb
Hg
h₂ = 86 cm
Z₁
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 5.19. The system in consists of a water reservoir with a layer of compressed air above the water and a large pipe and nozzle. The pressure of the air is 50 psig, and the effects of friction can be neglected. What is the velocity of the water flowing out through the nozzle? 50 psig 30 ft Flow driven by pressure against gravity.arrow_forwardThe pressure drop in a 6-in. pipe (I.D. = 6.065 in.) of length L is 1 psi with the flow in the highly turbulent region. If the same liquid flows at the same volumetric flow rate in a 3-in. pipe (I.D. = 3.068 in.) with length L, the pressure drop (psi) is:arrow_forwardquestion in imagearrow_forward
- Suppose that exactly half of the annular volume between concentric, horizontal cylinders is filled with a liquid and half with a gas, as shown below on the left. When the inner cylinder is rotated at an angular velocity , it is found that one interface rises to a height H above the other. The gap between the cylinders is sufficiently thin (W<< R) that local Cartesian coordinates can be used, as shown on the right. (For clarity, the gap width is exaggerated on the left.) All of the following questions concern flow in the liquid, which is Newtonian with constant properties. g R+W Gas Ω Liquid R H y Enlargement of liquid-filled gap X W ΩR Fixed (a) Assuming steady, fully developed flow, determine Vx(y) in the liquid in terms of dp/dx. (At this point, dp/dx is unknown.) (b) Relate dIP/dx in the liquid to H. You can assume that the absolute pressure in the gas is nearly constant (P = Po) and that surface tension is negligible. (c) Calculate H. That is, relate H to the rotation rate (),…arrow_forwardConsider an inverted manometer with oil of specific gravity 0.91, which is displayed in the image linked here. What is the difference in pressures between x and y in psi (pounds per square inch)?arrow_forwardquestion in imagearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The