
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row
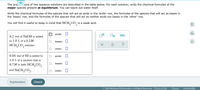
Transcribed Image Text:The pre, ons of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the
major species present at equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in
the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HCH, CO, is a weak acid.
圖
alo
acids:
0.2 mol of NaOH is added
to 1.0 L of a 0.5M
bases:
HCH,CO, solution.
?
other:
0.04 mol of HI is added to
acids:
1.0 L of a solution that is
0.7 M in both HCH,CO,
bases:
and NaCH,CO,.
other:
Explanation
Check
© 2021 McGraw-Hill Education. All Rights Reserved.
Terms of Use | Privacy I Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write an equation for the ionizations of carbonic acid H2CO3 a weak acid write the equilibrium constant experession for this acid ionizationarrow_forwardThe question is in the image. Additional information: In this explanation make sure to include either Le Chatelier’s Principle or equilibrium in the answer at the atomic level.arrow_forwardNonearrow_forward
- this one is hard bro, help.arrow_forwardder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select I next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of species 0.1 M aqueous solution H₂O HN3 C₂H5N 2 7 F N3 (Choose one) 1 (lowest) 234 OH C,HẠNH HF 5 6 + 7 8 (highest) 1 (lowest)arrow_forwardThe preparation of an aqueous solution is described in the table below. For this solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in. the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 0.07 mol of HNO3 is added acids: ☐ to 1.0 L of a solution that is 0.3M in both NH3 and NH₁Br. bases: ☐ ☐ other: ☐ 0,0,...arrow_forward
- Celery stalks that are immersed in fresh water for several hours become stiff. Similar stalks left in a 0.15 M salt solution become limp. From this we can deduce that the fresh water______.arrow_forwardDefine and give examples of conjugate acids and bases.arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of 0.1 M aqueous solution species H,0 (Choose one)Y HNO, (Choose one) Y HCOOH (Choose one) HCIO, (Choose one) Y H,0 1 (lowest) NO, HCO0 8 (highest) CIO,arrow_forward
- Please don't provide handwritten solution ...arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each spec Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next hig pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species CH₂CICOO HF F HNO₂ OH CH,CICOOH NO₂ H₂O relative pH of 0.1 M aqueous solution (Choose one) (Choose one) v 6 3 8 (highest) 1 (lowest) (Choose one) ▼ (Choose one) ▼ X Garrow_forwardFor each of the following entities • Identify it as a strong or weak acid. • Select the correct arrow to show the changes that occur when the acids are placed in water. • Select the approximate pH for each resulting solution. HCI(aq) is classified as a + acid. The reaction equation with water utilizes which of the following arrows? Choice A Choice B 7 >>7 H2SO3(aq) is classified as a The reaction equation with water would utilize which of the following arrows? Choice A Choice B < 50% H2SO3(aq) + H20(I) • HSO3 (aq) + H30*(aq) The approximate pH of the solution would bearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY