
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Try Again
Your answer is incorrect.
• Row 1: Your answer is incorrect.
Row 2: Your answer is incorrect.
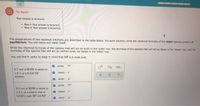
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
O acids:
HF
0.7 mot of KOH is added to
1.0 L of a 0.4M HF
9 bases:F
solution.
9 othert K
2 acids:
HF
0.3 mol of KOH is added to
O bases:F
1.0 L. of a solution that is
0.8M in both HF and KF.
other: K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Order these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species ΙΟ HF HIO F ОН NO₂ HNO₂ H₂O relative pH of 0.1 M aqueous solution (Choose one) (Choose one) 3 5 8 (highest) (Choose one) 2 (Choose one) X Śarrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species relative pH of 0.1 M aqueous solution + C6H5NH3 HCOOH 3 2 HCOO (Choose one) H₂O HONH3 5 + (Choose one) C6H5NH2 (Choose one) H₂O+ (Choose one) HONH2 8 (highest)arrow_forwardConstruct the expression for Ka for the weak acid, NH,. NH, (aq) + H,O(1)=H,0(aq) + NH,(aq) 1 Based on the definition of Ka, drag the tiles into the numerator or denominator to construct the expression for the given acid. Koarrow_forward
- First read the text sections 10.5 and 10.6 on pH. This is a Dry Lab since we cannot meet on Campus. After calibrating a pH meter you would have measured the pH of four different Basic Aqueous Solutions and examined the data for trends. You would have calculated the expected pH of each of the strong base solutions by assuming that for every NaOH formula unit that dissolves, one OH- ion is released. Thus, we can assume that the OH- Molarity equals the NaOH Molarity. Using the OH- Molarity and the water ionization equilibrium expression (Kw= 1.0 X 10 to the -14 power = H+ Molarity X OH- Molarity), we can calculate the H+ Molarity of the solution. Using the H+ Molarity, we can calculate the solution's pH. Thus a 1.0 X 10 to the 4 power NaOH solution has a pH of 10. The calculated pH value is arrived at as follows. By rearranging the water ionization equilibrium expression and plugging in the OH- Molarity, we can solve for H+ Molarity. H+ M = 1.0 X 10 to the -14 power divided by 1.0 X 10 to…arrow_forwardThe weak acid 0.250 M HNO2 is dissolved in water. HNO2(aq) ⇌H+ (aq) + NO2− (aq) . The resulting concentration of [H+] is 0.00253 M. Calculate the concentrations of [NO2− ] and [HNO2]. What is the freezing point?arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of 0.1 M aqueous solution species ? 103 |(Choose one) ▼ OH |(Choose one) ▼ H,0 4 HC,04 6. HIO, 1 (lowest) NO2 |(Choose one)arrow_forward
- The pOH of an aqueous solution at 25°C was found to be 3.20. The pH of this solution is The hydronium ion concentration is М. The hydroxide ion concentration is М.arrow_forwardDetermine the pH of each of the following solutions. 0.19 M CH3NH3I(Kb(CH3NH2)=4.4×10^−4) Express your answer to two decimal places.arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of species 0.1 M aqueous solution HC₂04 7 + H₂O* 1 (lowest) H₂PO4 (Choose one) H₂PO4 4 103 (Choose one) HIO₂ H₂C₂O4 (Choose one) H₂O (Choose one)arrow_forward
- 11 4000ol Ho00o 404 F-000HO HOO0HO HOOovale IHO 10H |-000Hal HO00H HO00'HO HO %3D ased on the definition of Ka, drag the tiles to construct the expression for the given acid. 1. CH,COOH(aq) + H,0(1) = H,O*(aq) + CH,COO-(aq) Construct the expression for Ka for the weak acid, CH,COOH. Question 26 of 27arrow_forwardIn 200 words or less, give an example of an acid or base found or used in the real-world. Include 2 uses, concentrations physical and chemical properties of the acid or base. Be sure to give some interesting facts.arrow_forwardThe basis for the pH scale is explained by: The intermolecular forces of water. The molecular polarity of water. The specific heat capacity of water. The molecular structure of water. The autoionization of water.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY