
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:M Inbox (44,3 x
w Virginia Cor X
Frcc Chapter 4 H x
Visual
session.masteringphysics.com/myct/itemView?assig
Apps
Getting Started
Thomson Reuters
o ADP ezLaborManag
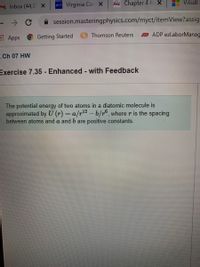
Ch 07 HW
Exercise 7.35 - Enhanced - with Feedback
The potential energy of two atoms in a diatomic molecule is
approximated by U (r) = a/r-b/r°, where r is the spacing
between atoms and a and b are positive constants.
Transcribed Image Text:Submit
Request Answer
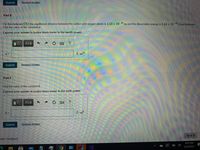
Part E
For the molecule CO, the equilibrium distance between the carbon and oxygen atoms is 1.13 x 10 10 m and the dissociation energy is 1.54 x 10 18 J per molecule.
Find the value of the constant a.
Express your answer in joules times meter in the twelth power.
να ΑΣφ
J.m12
Submit
Request Answer
Part F
Find the value of the constant b
Express your answer in joules times meter in the sixth power.
Eν ΑΣφ
b =
J.m
Submit
Request Answer
Next >
vide Feedback
6:20 PM
6/22/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- RTI The molecules in a six-particle gas have velocities ₁ (201-30j) m/s V2 (10i+60j) m/s 73=(-501 +20j) m/s 7= 30i m/s Vs (40i-40j) m/s ₁=(-50 i 10j) m/s Calculate Varg Express the x and y components of the velocity in meters per second separated by a comma. Uz avg, Uy avg Submit Part B VD|| ΑΣΦ Request Answer W Calculate Davg- Express your answer with the appropriate units. ? m/sarrow_forwardThe potential energy of two atoms in a diatomic molecule is approximated by U(r) = a/r12-b/r6, where r is the spacing between atoms and a and b are positive constants. Suppose the distance between the two atoms is equal to the equilibrium distance found in part A. What minimum energy must be added to the molecule to dissociate it - that is, to separate the two atoms to an infinite distance apart? This is called the dissociation energy of the molecule. Express your answer in terms of the variables a and b. For the molecule CO, the equilibrium distance between the carbon and oxygen atoms is 1.13\times 10-10m and the dissociation energy is 1.54\times 10-18J per molecule. Find the value of the constant a. Express your answer in joules times meter in the twelth power. Find the value of the constant b. Express your answer in joules times meter in the sixth power.arrow_forwarda) Estimate the average spacing between the molecules of 1 mol of an ideal gas at a pressure of 1atm and a temperature of 300 K. b) 1 mol of liquid water occupies a volume of 18 cm. Estimate the spacing between molecules. c) Use your result from part (a) to estimate the diameter of a water molecule. d) Estimate the factor by which water expands when it boilsarrow_forward
- How would I draw the following graphs for a simple ohmic device from the equations V=IR and P=IV=I^2R=V^2/R: plot V vs. I with constant R, plot P vs V with constant R, plot P vs R with constant I, and plot P vs I with constant R.arrow_forwardIn the Taylor Expansion, it looks like they've made x_0 = x_1, x_2 and made x = x_1 - a, x_2 - a. I don't understand how they can use that substitution for x_0 since it is a constant, and x_1 and x_2 are variables that can change.arrow_forward2. For T = 300 K, calculate the pressure (in bars) at which the mean free path of a hydrogen molecule will be each of the lengths given here. For H₂, o = 2.30 x 10-1⁹ m². (a) 100 μm (b) 1.00 mm (c) 1.00 m LLarrow_forward
- The temperature of an ideal monatomic gas is increased from 25 C to 50 C. Does the average translational kinetic energy of each gas atom double? Explain. If your answer is no, what would the final temperature be if the average translational kinetic energy was doubled?arrow_forward8. The average energy of a mole of Einstein solid is given by: ӨЕ 3R0E Ē = 3R + 2 ӨЕ ет — 1 where E is the Einstein temperature and R the gas constant. Obtain expressions for the average energy at the limits of zero and infinite temperature and comment on your results.arrow_forwardA container of nitrogen molecules is at a temperature of 37.0°C. What is the mass of a nitrogen molecule in atomic mass units? [Note: nitrogen is a diatomic gas.] а. b. What is the mass of a nitrogen molecule in kilograms? C. What is the average translational kinetic energy of the nitrogen molecules? d. What is the rms speed of the nitrogen molecules? е. What is the average rotational energy of a nitrogen molecule?arrow_forward
- What is the average kinetic energy of a proton in the center of the sun, where the temperature is 2.0 x 107 K? Express your answer with the appropriate units. Eavg Submit Part B μА Value Request Answer [www] Units ? What is the rms speed of a proton in the center of the sun, where the temperature is 2.0 x 107 K? answer with the appropriate units.arrow_forwardA molecule in Earth's atmosphere at 20 °C has a most probable energy E = kT/2 = 2.02 ×10-21 J E = kT =4.04 ×10-21 J E = 3 k T / 2 = 6.1 ×10-21 J that depends on the mass of the moleculearrow_forwardFor the given A→ draw B→ that will guarantee C = 0.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON