
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
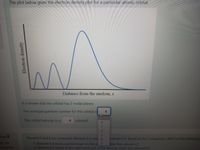
Transcribed Image Text:The plot below gives the electron density plot for a particular atomic orbital.
Distance from the nucleus, r
It is known that the orbital has 2 nodal planes.
The principal quantum number for this orbital is
+1
This orbital belongs to a
subshell
1.
2.
estion 2
Element Z and X are compared. Element Z is sma 4
element X Based on this comparison, which of the following
ot yet
mswered
1. Element X is from a period lower on the pi
2. Element Z is closer to the right hand side of the periodic table than element X
ble than element 2
Electron density
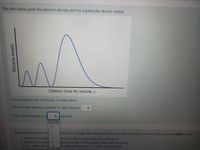
Transcribed Image Text:The plot below gives the electron density plot for a particular atomic orbital.
Distance from the nucleus,
It is known that the orbital has 2 nodal planes.
The principal quantum number for this orbital is
This orbital belongs to a
subshell
1n
Element Z and X are com
lement Z is smaller than element X. Based on this compaison, which of the following could be true?
1. Element X is from g
2. Element Z is closer f
3. A 1+ cation ion of
4 Flement 7 bas a higher
lower on the periodic table than element Z
ight hand side of the periodic table than element X
X will be larger than a 1+ cation of element 2
of
Electron density
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the number of s, p, d, and f electrons in each of the following atoms. (Please remember that Cu and Cr have irregular electron configurations. Also, the total number of electrons should add up to the atomic number of the neutral atom.) d atom Cd (Z=48) Fr (Z=87) Cu (Z=29) Рarrow_forward11arrow_forwardAn experiment was conducted to determine IE1 for magnesium. In this experiment it was determined that 1.193 x 10-18 J was needed to remove the first electron from an atom of magnesium in the gas phase. According to these experimental results, what is the first ionization energy of magnesium?arrow_forward
- 2) The ground-state valence-shell configuration of a particular atom is 4s23d104p5. Theelement to which this atom belongs is a? noble gas. Halogen alkali metal transition metal metalloidchalcogen alkaline earth metal actinide lanthanidearrow_forward4. Calcium has 20 electrons in total. The ionization energy for the first 18 have been graphed below. Provide appropriate labels for the two empty boxes. IONISATION ENERGY (kJ mol¹) 120000 100000 80000 60000 40000 20000 O IE3: REMOVAL OF ELECTRON FROM 3p 1 2 3 IE 17: REMOVAL OF ELECTRON FROM 2s 4 5 6 7 NUMBER OF ELECTRONS REMOVED 8 9 10 11 12 13 14 15 16 17 18arrow_forwardPredict the electron configuration for the C atom: Predict the electron configuration for the P atom: Predict the electron configuration for the V atom: Predict the electron configuration for the Sb atom: Predict the electron configuration for the Sm atom: Predict the electron configurations of N3– Predict the electron configurations of Ca2+ Predict the electron configurations of S2– Predict the electron configurations of Cs1+ Predict the electron configuration of Cr2+ Predict the electron configuration of Gd3+arrow_forward
- The electron configuration of an element is: 1s? 2s? 2p6 3s? 3p6 4s? 3d10 4p6 5s2 The Group and Period of the element are: Group 5 Period 1A Group 2B Period 5 Group 5 Period 1B Group 2A Period 5arrow_forwardAtoms of two adjacent elements in the periodic table have a total of 40 d electrons. Atom A has no p electrons in its valence shell but atom B has one electron in the p subshell of its valence shell. Element A is_ and element B is _arrow_forwardI am confused. It states that Nobel gasses have zero valence electrons. Then it says that group 8 elements have 8 valence electrons but group 8 are noble gases. How is this possible or am I wrong altogether? Which elements have zero valence electrons?arrow_forward
- Give the indicated full or noble gas electron configuration Note: you can't do superscripts in questions answers, so format your answer without superscripts but with one space between each orbital. E.g. Li would be 1s2 2s1 instead of 1s22s The number in parentheses after the symbol is the element's atomic number. Full configuration: N (7) Full configuration: Si (14) Full configuration: Ti (22) Noble gas configuration: Ba (56) ( Noble gas configuration: Te (52) (99+ hparrow_forwardplease solve question 1 and 2, thanks alot sirarrow_forwardAn atom with the element symbol X has 62 protons in its nucleus. For the 62nd electron, determine the ionization energy of the electronarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY