
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:ny
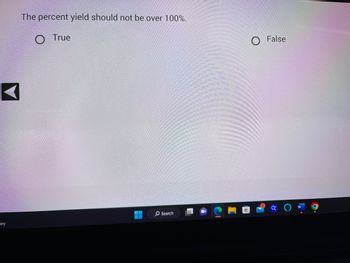
The percent yield should not be over 100%.
O True
O Search
O False
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H₂O). If 1.90 g of water is produced from the reaction of 35.6 g of hydrobromic acid and 7.39 g of sodium hydroxide, calculate the percent yield of water. Round your answer to 3 significant figures. ☐% Xarrow_forward1) The Law of Conservation of Mass states that the total mass, in an open system, does not change as the result of reactions between its parts. True False 2) The deviation from the theoretical yield to the actual yield is called the percent yield. True Falsearrow_forward+ 1 0.209 0.418 Determine the maximum number of grams of product that can be produced in the balanced reaction described below by constructing a BCA table and calculating the maximum grams of product. Complete Parts 1-2 before. submitting your answer. Fe,O,(s) + 3 CO(g) → 2 Fe(s) + 3 CO₂(g) 1 2 NEXT> A reaction occurred starting with 33.4 g of iron(III) oxide mixed with 47.2 g of carbon monoxide. Based on your knowledge of stoichiometry, set up the table below to determine the amounts of each reactant and product after the reaction goes to completion. Before (mol) (mol) After (mol) 0 -0.209 -0.418 Question 33 of 57 FeO,(s) + 3 CO(g) 33.4 0.296 1.06 -33.4 -0.296 -1.06 1 47.2 1.69 2 Fe(s) + 3 CO (9) -47.2 -1.69 1.19 0.627 1 -1.19 RESET -0.627 ( ** Submitarrow_forward
- DDT, which can be prepared by the reaction shown below, was an insecticide used to prevent mosquito borne diseases. Due to the harmful effects on birds and fish, it is now banned in the US. However, other countries still use it to control mosquitos. Determine the amount of DDT that can be produced and the percent yield for this reaction by constructing a BCA table, determining the maximum grams of DDT that can be produced, and calculating the percent yield. Complete Parts 1-3 before submitting your answer. 1 Before (mol) Change (mol) After (mol) 2 C₂H₂CI+ C₂HOCI → C₁H₂Cl + H₂O 3 14 9 5 2 3 NEXT > If a company in South America started with 1175 g of chlorobenzene (CH₂CI, MW 112.55 g/mol) and 538.5 g of chloral (C₂HOCI, MW 147.39 g/mol), set up the table below that represents 100% yield with the given reaction conditions. 2 C₂H₂CI + C₂HOCI CH.CI 14 9 5 + H₂Oarrow_forwardNeed help with percent composition calculation and empirical formulaarrow_forwardMolar Masses A = 256.03– E = 157.45mole D = 194.68 X = 276.25mole mole mole G = 192.47mole Z = 162.05–9 mole If 13.41 g A are reacted with 12.53 g D, according to the balanced chemical equation below, how many grams of G will be produced? 4 A + 3 D – 6 Z + 6 G Please input your answer to the third decimal place Type your answer...arrow_forward
- TheoreticalYield=1.96g Actual=1.78g %Yield=____________arrow_forwardD 0/3 Percent yield of chemical reactions Liquid octane (CH;(CH,) CH,) reacts with gaseous oxygen gas (O,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). If 28.5 g of carbon dioxide is produced from the reaction of 11.4 g of octane and 55.6 g of oxygen gas, calculate the percent yield of carbon dioxide. Round your answer to 3 significant figures.arrow_forwardThe theoretical yield of product for a particular reaction is 37.06 g. A very careless student obtained only 35.47 g of product after carrying out this reaction. What is the percent yield that this student obtained? Enter a number only; do not spell Include the correct number of significant figures for your answer Answer: % yieldarrow_forward
- Gaseous methane CH4 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. What is the theoretical yield of water formed from the reaction of 0.80g of methane and 1.6g of oxygen gas? Be sure your answer has the correct number of significant digits in it.arrow_forwardAn ethanol plant begins a production run with sufficient reactants to predict a theoretical yield of 1250 metric tons of ethanol. The process produces 1178.6 metric tons. What is the percent yield for this production run?arrow_forwardA 60.5 mL sample of a 0.104 M potassium sulfate solution is mixed with 35.5 mL of a 0.100 M lead (II) acetate solution and the following precipitation reaction occurs: K2SO4 (aq) + Pb(C2H3 O2)2 (aq)→2KC₂ H3 O2 (aq) + PbSO4(s) The solid PbSO4 is collected, dried, and found to have a mass of 0.993 g. Determine the limiting reactant, the theoretical yield, and the percent yield.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY