
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The peak intensity of the CMBR occurs at a wavelength of 1.1 mm.
(a) What is the energy in eV of a 1.1-mm photon? (b) There are approximately 109 photons for each massive particle in deep space. Calculate the energy of 109 such photons. (c) If the average massive particle in space has a mass half that of a proton, what energy would be created by
converting its mass to energy? (d) Does this imply that space is “matter dominated”? Explain briefly.
Expert Solution
arrow_forward
Part (a)
The wavelength,
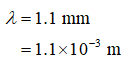
arrow_forward
Step 2
Planck’s constant,
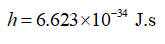
arrow_forward
Step 3
The speed of light,
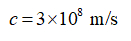
arrow_forward
Step 4
The energy in eV,
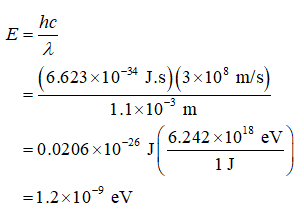
arrow_forward
Step 5
The energy in eV of a 1.1-mm photon,
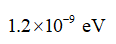
Step by stepSolved in 10 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Under the right circumstances, a photon of high enough energy can give rise to an electron-positron pair. What minimum energy photon is required?arrow_forwardAs noted in the chapter, the cosmic microwave background radiation fits the Planck equation for a blackbody at 2.7 K. (a) What is the wavelength at the maximum intensity of the spectrum of the background radiation? (b) What is the frequency of the radiation at the maximum? (c) What is the total power incident on Earth from the background radiationarrow_forwardAn electron in the n = 9 energy level of hydrogen undergoes a transition to the n = 5 energy level. Determine the energy in eV, the energy in joules, and the frequency of the emitted photon (a) the energy in eV (eV) (b) the energy in joules (J) (c) the frequency of the emitted photon (Hz)arrow_forward
- When you shine a certain source of EM radiation on a sheet of gold (work function W1 = 5.1 eV), the stopping voltage required to bring all the ejected electrons to a halt is V = 3.5 V. If you were to use this same source of EM radiation on a sheet of potassium (work function W2 = 2.3 eV), what would be the maximum speed of ejected electrons?arrow_forwardI'm still confused about the step 2 part. How would I put this into my calculator? Where do the extra numbers 232.6 come from?arrow_forwardI need help trying to figgure out how to fill out the graph by just using the info on the grapharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON