
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
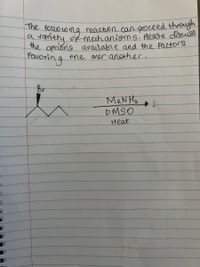
Transcribed Image Text:he fouocoing recehon can proceed through
vatehy og mech anioms. lease disuiss
the optons avaitable and the Factors
a
Favoring
one arer another.
MeN Hz
DMSO
Heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Vames and Ti... V At Your Service Inte... Sy log_{b}sqrt(b) - Sim... O (Last Day Promotio.. Kronos Workforce... Maame Acheampo.. MIDTERM MODER... [References] In a coffee-cup calorimeter, 100.0 mL of 1.1 M NAOH and 100.0 mL of 1.1 M HCl are mixed. Both solutions were originally at 20.3°C. After the reaction, the final temperature is 27.7°C. Assuming that all the solutions have a density of 1.0 g/cm" and a specific heat capacity of 4.18 J/°C g, calculate the ethalpy change for the neutralization of HCl by NaOH Assume that no heat is lost to the surroundings or to the calorimeter. AH= kJ/mol Submit Answer Try Another Version 1 item attempt remaining Previous Next, 8:31 PM 1へ梦口后会 12/5/2020 hingarrow_forward4) Synthesis of ammonia by the Haber process occurs by the reaction N22) + 3H2g) 5 2NH3(g) Species AH (kJ mol"') 4S° (J mol- K-') H2(g) 0. 130.7 N2(8) 0. 191.6 NH3(8) -46.1 192.5 Assuming that AH° and AS° are essentially unchanged in the temperature ranging from 25 °C to 400 °C: a. Calculate K at 25 °C. b. Calculate K at 400 °C. (R= 8.314 J mol- K')arrow_forwardes Mailings Review View O Tell me 三 三 AaBbCcDdEe AaBbCcDdEe AaBbCcDdEe AaB Normal Body Text No Spacing Table Paragr... Не 5, and improvements, choose Check for Updates. Answer the following questions. CO(g) +2 H2(g) CH3OH(1) AH° =-128.1 kJ S° AH (kJ mol-') -110.5 -238.6 AG (kJ mol-1) (J mol- K-) CO(g) CH3OH(1) -137.3 -166.2 +197.9 +126.8 The data in the table above were determined at 25°C. (a) Calculate AG° for the reaction above at 25°C. (b) Calculate Keg for the reaction above at 25°C. (c) Calculate AS for the reaction above at 25°C. (d) In the table above, there are no data for H2. What are the values of AHƐ, AGƐ, and of the absolute entropy, S°, for H2 at 25°C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY