
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The normal freezing point of a certain liquid X is -2.6°C, but when 59. g of urea ((NH2)2CO) are dissolved in 550. g of X, it is found at the solution freezes at -6. Zero instead uses information to calculate the molal freezing point depression of Kf of X and round your answer to 2 sig figs. 
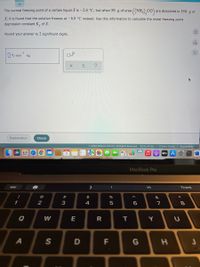
Transcribed Image Text:The normal freezing point of a certain liquid X is –2.6 °C, but when 59. g of urea
CO are dissolved in 550. g of
X, it is found that the solution freezes at -6.0°C instead. Use this information to calculate the molal freezing point
depression constant K, of X.
圖
Round your answer to 2 significant digits.
do
1
°C-mol
· kg
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center | Accessibility
étv
MacBook Pro
esc
Hi
Thank
@
#
$
*
1
2
3
4
7
Q
W
E
R
Y
A
S
D
F
T
Expert Solution
arrow_forward
Step 1

Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain liquid x has a normal freezing point of 1.30 °C and a freezing point depression constant K,- 3.30 °C-kg'mol!. A solution is prepared by dissolving some glycine (c,H,NO,) in 850. g of x. This solution freezes at - 2.2 °C. Calculate the mass of c,H,NO, that was dissolved. Be sure your answer is rounded to the correct number of significiant digits. ?arrow_forwardA (non-aqueous) solution is found to have a molality of 2.42 molal and experiences a freezing point depression of 6.50 degrees C. What is the Kf of the solvent (in degrees C/m)?arrow_forwardIf dissolving 1.5 g of a solute into 100 mL of water caused the temperature of the solution toincrease by 4.7 ⁰C, what would the change of temperature be if 3.0 g of the solute were dissolvedin the same volume of water? Explain.arrow_forward
- An experiment was conducted to determine the effect of glucose on the freezing point of water. Experimental data showed thatthe freezing point depression of water in solution was –2.6°C when 1.0 g of glucose was dissolved in 10g of solvent. Calculatethe expected freezing point for such solution and compare the expected freezing point to the value found experimentally. Give aplausible explanation for any discrepancies. (M.W. of glucose= 180.16g/mol; i=1). Show all your work.arrow_forwardGiven the following mixture of two compounds 45.00 mL of X (MW =80.00 g/mol)(density 0.893 g/mL) and 885.00 mL of Y (55.00 g/mol))(density 1.280 g/mL). The freezing point of pure Y is 54.00 degrees C. The molal freezing constant is 4.757 degrees C/m. What is the freezing point of the solution.arrow_forwardWith correct sig figsarrow_forward
- When 127. mg of a certain molecular compound X are dissolved in 55.0 g of dibenzyl ether point of the solution is measured to be 1.6 °C. Calculate the molar mass of X. If you need any additional information on dibenzyl ether, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. ((C6H₂CH₂)₂0), 2 the freezing OO B olo Xuarrow_forwardIf dissolving 1.5 g of a solute into 100 mL of water caused the temperature of the solution toincrease by 4.7 ⁰C, what would the change of temperature be if 3.0 g of the solute were dissolvedin the same volume of water? Explain.arrow_forwardHelp with the following questionarrow_forward
- A sports drink formulation can be prepared by mixing the ingredients in the quantities shown in the table below. Calculate the freezing point of this drink formulation. (Kffor water is 1.86 °C kg mol-1 and the molar mass of sucrose is 342.3 g mol-¹). Ingredient Mass (g) NaCl 1.2 0.49 KCI Sucrose (C12H22011) 60 1000 water O a. -0.38 °C O b.-0.43 °C O c. -0.10 °C O d. -0.32 °C O e. -0.75 °C Which ONE of the following saturated aqueous salt solutions will contain the highest total concentration of ions at 25°C? a. AgCl = 1.8 x 10-10) (Ksp (Ksp = 3.9 × 10-11) O b. CaF2 OC. Ag2CO3 O d. COCO3 (Ksp (Ksp = 1.0 x = 8.1 x 10-12) 10-10) The highest amount of solid Cul2 will dissolve in 1.0 L of an aqueous solution of: a. 0.5 M CaCl2 b. 0.5 M Cu(NO3)2 O c.0.5 M Cul2 O d. 0.5 M NH4I O e. 0.5 M CuCl2arrow_forwardWhen 52.3 g of alanine (C;H,NO,) point of pure X. On the other hand, when 52.3 g of iron(III) nitrate (Fe(NO;),) are dissolved in 600. g of a certain mystery liquid X, the freezing point of the solution is 3.3 °C lower than the freezing are dissolved in the same mass of X, the freezing point of the solution is 4.3 °C lower than the freezing point of pure X. Calculate the van't Hoff factor for iron(III) nitrate in X. Be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits. i =arrow_forwardWhen 4.59 g of a certain molecular compound X are dissolved in 35. g of dibenzyl ether ((C%H,CH,),°). the freezing point of the solution is measured to be 0.9 °C. Calculate the molar mass of X. If you need any additional information on dibenzyl ether, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to 1 significant digit. 0 00 0.0 X x10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY