The molecular binding energy is called the dissociation energy. It is the energy required to separate the atoms in a molecule. The dissociation energy of the NaCl molecule is 4.24 eV. Determine the fractional mass increase of the Na and Cl atoms when they are not bound together in NaCl. What is the mass increase for a mole of NaCl?
Q: Two plastic rulers (R1 and R2) were subjected to the same stress. Whereas R1 extended by only 10% of...
A: Subpart (A): Young's modulus of any material is defined as the ratio of the longitudinal stress to t...
Q: Suppose you could measure the rate constants for both the forward and reverse reactions of the proce...
A: Given: To explain the direction of the rate constant in which will be a larger as,
Q: How many moles of an ideal gas exert a gauge pressure of 0.876 atm in a volume of 5.43 L at a temper...
A: PV=nRTn=PVRT=0.876×5.430.0821×295.2
Q: Suppose 10.0 kV is applied on a 3.54-nF parallel-plate capacitor. How many microcoulombs are in each...
A: Given Voltage (V) = 10.0 KV = 10×103 V Capacitor (Q) = 3.54 nF = 3.54×10-9 C/V
Q: How much heat energy in kJ is required to melt a 45kg of ice?
A: Here, we use the latent heat of ice to calculate the total heat required to melt the given amount of...
Q: #6. One the following vector fields F(x,y,z)= xyz*i +x²z*j+4x²yz³k G(x, y,z)=i+ (sin z) j + y (cos z...
A:
Q: (Astronomy) Neutron Star Angular Size. Part A: If Earth's Moon were replaced by a typical neutron s...
A: Part (A) The formula to calculate the angular diameter of neutron star seen from earth is, δ=dD Here...
Q: Calculate the radiation dosage and grace for a 57-kg person Who is exposed for 8.0 as to a 3.0 Ci so...
A:
Q: An electric motor consumes 9.00 kJ of electrical energy in 1.00 min. If one-third of this energy goe...
A: Given: To find the torque of the engine in which run as,
Q: Given the work functions for some metals:- Aluminum=4.08eV, Beryllium=5.0eV, Cadmium=4.07eV, Calcium...
A: The above problem can be saved by using the concept of photoelectric effect as follows
Q: The fraction of defective integrated circuits produced in a photolithography process is being studie...
A: Given The fraction of defective integrated circuits produced in a photolithography process is being ...
Q: Is the photoelectric effect in contrast to classical theories? Yes, counterintuitive that energy of ...
A:
Q: Advanced Physics Question
A: This problem can be solved using the for for the fraction of an object floating over a liquid obtain...
Q: An n-type semiconductor sample is continuously and uniformly excited (optically) at low level. Start...
A: please see the next step for solution
Q: High-energy subatomic particles coming from space interact withatoms in the earth’s upper atmosphere...
A: The Problem is a basic Problem of time dialation The Dialated time is t=2.20 x 10-6 s , as measured...
Q: A naturally occuring gold crystallizes in face centred cubic structure and has a density of19.3 g/cm...
A: Given data, Density of gold = 19.3 gm/cm3 Atomic weight = 197 gm/mole
Q: It has been observed that the potential function; V (x, y, z) = - 1 V in the region x <-10V (x, y...
A: As we know, ∇×E =0This equation indicates that an electric field is conservative.Therefore, for E we...
Q: Q2 If one uses an electric conductor that is much thicker than the skin depth, 8, conductive materia...
A: please see the next step for solution
Q: In the film The Italian Job, a coach is balancing on the edge of a cliff with gold bullion at one en...
A:
Q: A glucose solution of viscosity 2.2 x 10-3 pascal-second and of density 1.03 kg/L flows from elevate...
A: Given, Fluid viscosity, v=2.2×10-3 pascal-second Density, d=1.03 kg/L⇒d=1.030.001 kg/m3⇒d=1030 kg/m...
Q: Can you solve it plz its material science and also can you explain each steps briefly
A: Given:- Density of Gold=19.3 g/cm3 atomic weight =197 g/mol
Q: Show your solution. The final answe is 48km/hr.
A: Let total distance be, 2d. For 1st half, distance be 'd'. Time t1. Initial velocity = 40 km/h; fina...
Q: Need help with the attached question, please help with all parts of the question.
A: When the randomly polarized light is incident on first polarizer, the intensity of light coming out ...
Q: Suppose you repeated Rutherford’s scattering experiment with a thin sheet of solid hydrogen in place...
A: Suppose you repeated Rutherford’s scattering experiment with a thin sheet of solid hydrogen in place...
Q: Using Bohr's model, calculate the velocity of the electron orbiting on a stable trajectory with radi...
A: Using Bohr's model The velocity of the electron orbiting in nth orbit is given by Where z = atomic...
Q: a) Find the mathematical expression for the voltage VR (t).b) Calculate the reactive power on the ca...
A: (a)XL =jωL =5000*400μ =2 ohmApplying KVL in the left half loop of the circuit VR(t) =1C∫i3(t)dt +VS2...
Q: An insect of mass m walks with constant speed v0 in a circular path of radius b on a rotating disk w...
A: The formula to calculate the acceleration of insect is, r→''=a→'+ω˙×r→+2ω→×r→'+ω→×ω→×r→+A0 Here, r→'...
Q: How much heat (in Joules) does it take to raise the temperature of 100 g of H2O from 22 ̊C to 98 ̊C?
A: The amount of heat required to increase of unit amount of a substance by unit amount of temperature ...
Q: What is the probability that an electron in the 3d state is located at a radius greater than a0?
A: Given:- To find the probability that an electron in the 3rd state is located at a radius greater th...
Q: Which of the following statements is NOT directly from one of the five postulates in quantum mechani...
A: According to postulates of quantum mechanics.
Q: Since the potential of a perfect conducting sphere with a radius of 3 cm in empty space is 8 V, calc...
A: r=19.1 cm = 0.191 m Vo=8 V R=3 cm = 0.03 m charge Q is calculated using the formula V=kQ/d Q=Vd...
Q: Three horizontal ropes pull on a large stone stuck in the ground, producing the vector forces A vec ...
A: From the given data:We can consider the fig.1 :Now,Here we can add the vectors :The x sum of the vec...
Q: Show that the Planck radiation law agrees with the RayleighJeans formula for large wavelengths.
A: Rayleigh- Jeans formula :- According to this formula, total energy density in a cavity in the freque...
Q: 03: There is threshold voltage in GM counter but in ionization chamber no threshold is required why?
A: In the Ionisation chamber usually, ionize the radiation from the source creates an ionization of the...
Q: Using Bohr's model, calculate the radius of the stable electron orbit with main quantum number 1 for...
A: n= 1 Z= 3
Q: In an L-R-C series circuit the magnitude of the phaseangle is 54.0°, with the source voltage lagging...
A: In a LCR circuit, Inductive reactance is give by XL=Lω
Q: Please be quick.
A:
Q: Q5: Assuming that the linear momentum of a given particle can be measured with an accuracy of 10⁻³ ...
A: 1. Momentum is, p=mv=5×10-3 kg2 m/s=10-2 kgm/s
Q: A series circuit has an impedance of 60.0 Ω and a power factor of 0.720 at 50.0 Hz. The source volta...
A: Impedance is given by, Z=R2+(XL-XC)2 XC is the capacitive reactance, XL is the inductive reactance a...
Q: An inductor with an inductance of 2.50 H and a resistance of 8.00 Ω is connected to the terminals of...
A: Given data L=2.5 HR=8 Ωε=6 V
Q: A 3.00 kg box that is several hundred meters above theearth’s surface is suspended from the end of a...
A: Given that,Mass of the box (m) = 3 kgTension in the rope T(t) = 36 N/sec (t)Here we need to find the...
Q: Why does the atomic number Z determine the chemical properties of a nuclide? What differences in che...
A: The atomic number is very useful to determine the chemical properties of a nuclide. The atomic numbe...
Q: A copper atom has lost five electrons. Calculate the net charge of the atom. -4.806x10-19 ...
A: Solution: As per the given data,
Q: Suppose that the central diffraction envelope of a double-slit diffraction pattern contains 19 brigh...
A: please see the step for solution
Q: A laser beam travels from air (n=1) into glass (n=1.5) and then into gelatin. The incident ray makes...
A: please see
Q: Cut off says during that time the friction from the tires. Ignore the numbers in the first sentence ...
A: According to Newton's second law, the forces always occur in pairs, that is to an applied force ther...
Q: A radio tune circuit consists of an inductor of inductance 0.4 H and resistance 10 Ω connected in pa...
A: Answer:
Q: A stuntman drives a motorcycle around a circular vertical wall 100ft in diameter. The coefficient ...
A: The circular wall has a diameter of 100 ftSo, the radius of this wall will be 50 ftLet r=50 ftr=50 f...
Q: A glucose solution of viscosity 2.2 x 10 -3 pascal-second and of density 1.03 kg/L flows from elevat...
A: Given data: Viscosity of fluid is 2.2×10-3 p.s=2.2×10-3 N-s/m2=η Density is 1.03 kg/L ==1.030.001 kg...
Q: How did we calculate terminal velocity v(m/s) = 0.03763? Note that inner radius = 0.015 m
A: Let d be the distance moved by the glass bead. Velocity is, V=dt
The molecular binding energy is called the dissociation energy. It is the energy required to separate the atoms in a molecule. The dissociation energy of the NaCl molecule is 4.24 eV. Determine the fractional mass increase of the Na and Cl atoms when they are not bound together in NaCl. What is the mass increase for a mole of NaCl?
(a)
Let E denotes the energy, c denotes the light speed, and Δm denotes the mass increased when separated. Therefore, the mass increased can be determined by using the mass-energy equation as,
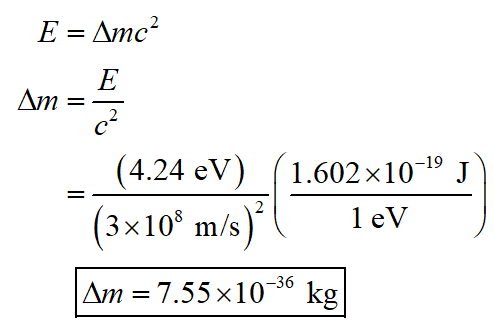
Step by step
Solved in 2 steps with 2 images
