
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
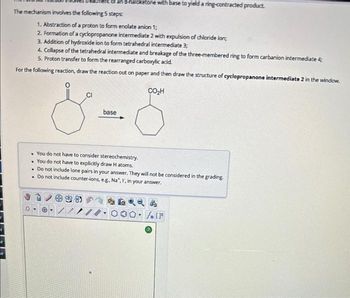
Transcribed Image Text:R
The mechanism involves the following 5 steps:
haloketone with base to yield a ring-contracted product.
1. Abstraction of a proton to form enolate anion 1;
2. Formation of a cyclopropanone intermediate 2 with expulsion of chloride ion;
3. Addition of hydroxide ion to form tetrahedral intermediate 3;
4. Collapse of the tetrahedral intermediate and breakage of the three-membered ring to form carbanion intermediate 4;
5. Proton transfer to form the rearranged carboxylic acid.
For the following reaction, draw the reaction out on paper and then draw the structure of cyclopropanone intermediate 2 in the window.
CO₂H
CI
8 5
base
. You do not have to consider stereochemistry.
. You do not have to explicitly draw H atoms.
. Do not include lone pairs in your answer. They will not be considered in the grading.
. Do not include counter-ions, e.g., Na, I, in your answer.
*****
O. Sn [F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The mechanism for the base-catalyzed hydration of a ketone begins with O protonation of the carbonyl oxygen Ohydroxide attack at the carbonyl carbon Oprotonation of the carbonyl carbon Ohydroxide attack at the carbonyl oxygenarrow_forwardIn a mixture of the two reactants, HNO3 and methyl benzoate, the nitration of methyl benzoate O Would form primarily methyl para-nitrobenzoate. O Would form primarily methyl meta-nitrobenzoate. O Would not take place. O Would form primarily methyl ortho-nitrobenzoate.arrow_forwardWhy are the carboxylic acid groups added in a syn orientation during hydrolysis for the following reaction?arrow_forward
- 4. Write the reagents and steps needed to perform the following synthesis (multiple steps may be needed). NH₂ COOHarrow_forwardFill in the blanks in the following reaction scheme. OH 1) MCPBA NaNH, 1) NaOH 2) H₂O+ 1) TMS-Cl in TEA CI OH Major Product 1)Trimethylsilyl chloride Triethylaminearrow_forward18) The following reaction undergoes three distinct steps: imine formation, electrophilic aromatic substitution, and decarboxylation. Draw each of the intermediates from each of these three steps as well as the final product. BS NH₂ OH heatarrow_forward
- This is the structure of xylan given, is the mechanism still the same (would there be two hydrolysis steps)?arrow_forwardTamoxifen is a drug used to prevent and treat breast cancer. What are all the possible metabolic reactions for this drug? Aromatic hydroxylation Epoxidation ON-dealkylation Benzylic hydroxylation O Epoxidation followed by epoxide hydrolysisarrow_forwardProstaglandins are a class of cicosanoids, fatty acid derivatives with a variety of extremely potent actions on vertebrate tissues. They are responsible for producing fever and inflammation and its associated pain. Prostaglandins are derived from the 20- carbon fatty acid arachidonic acid in a reaction catalyzed by the enzyme prostaglandin endoperoxide synthase. This enzyme, a cyclooxygenase, uses oxygen to convert arachidonic acid to PGG2, the immediate precursor of many different prostaglandins. Rate of formation of PGG2 with 10 mg/ml ibuprofen (mM/min) Arachidonic acid (mM) Rate of formation of PGG2 (mM/min) 0.190 12.3 0.228 13.9 0.342 17.5 0.570 1.33 22.2 28.8 7.71 8.88 11.9 16.3 24.0 The kinetic data given in the table are for the reaction catalyzed by a mutant of prostaglandin endoperoxide synthase. Focusing here on the first two columns, determine the Vmax and Km of the enzyme. Vmax = Km mM/min mMarrow_forward
- how to synthesize 2-phenylclohexanone from cyclohexanone?arrow_forward1. NaOEt CO₂Et 2. H3O+ 3. heat Br H3C CO₂H CO2 H3C CO₂Et The malonic ester synthesis is a carbonyl alkylation reaction. It is used to prepare carboxylic acids from primary alkyl halides, lengthening the carbon chain by two atoms. Thus, the product can be visualized as being a "substituted acetic acid." The reaction consists of three steps: generation of the enolate anion followed by SN2 reaction with a primary alkyl halide, ester hydrolysis under acid conditions, and decarboxylation. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions 22 CX EtO₂C EtO₂C HOCH2CH3 HOCH2CH3 EtO₂C EtO₂Carrow_forwardWhat is the major product when benzene reacts with Acetyl Chloride in AICI3 ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY