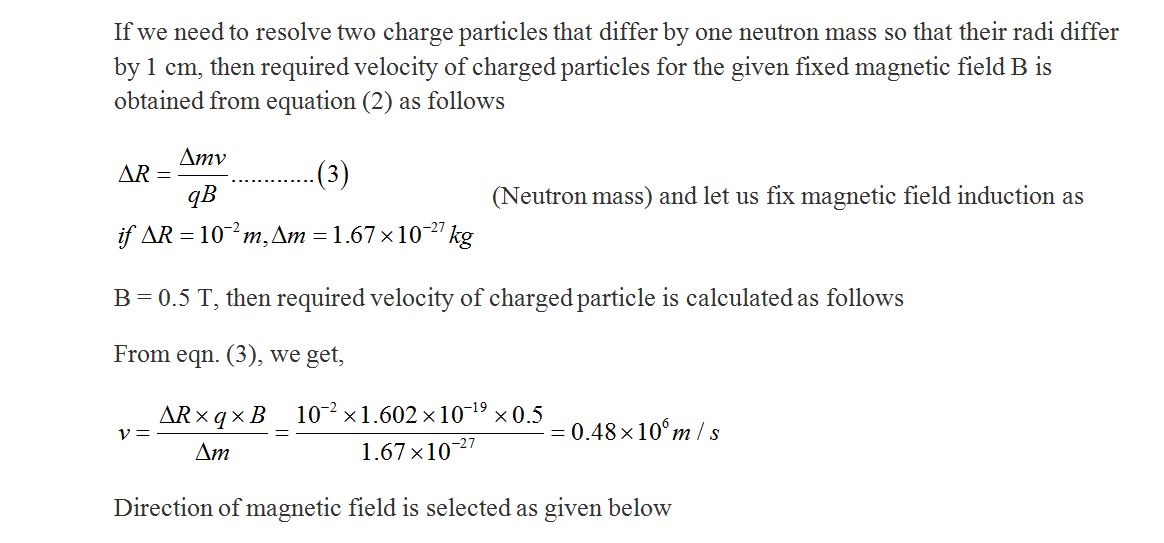
The mass difference between two isotopes is sometimes just a neutron mass. The spectrometer should separate them very well. For such an isotope combination, the difference in radius should be around 1 cm. That is r, - r = 1 cm. In order to achieve this, choose a magnetic field with a magnitude in Tesla (maximum magnetic field you can obtain from a conventional magnet is around 2.5 T so be far away from this value) and choose the direction also. Then determine the velocity of isotope you need. Last calculate radius r of a Pbz04 smallest isotope.
The mass difference between two isotopes is sometimes just a neutron mass. The spectrometer should separate them very well. For such an isotope combination, the difference in radius should be around 1 cm. That is r, - r = 1 cm. In order to achieve this, choose a magnetic field with a magnitude in Tesla (maximum magnetic field you can obtain from a conventional magnet is around 2.5 T so be far away from this value) and choose the direction also. Then determine the velocity of isotope you need. Last calculate radius r of a Pbz04 smallest isotope.
Related questions
Question
100%
Hello, Can you help me for question 1?
Thank you.


Transcribed Image Text:The mass difference between two isotopes is sometimes just a neutron
mass. The spectrometer should separate them very well. For such an
isotope combination, the difference in radius should be around 1 cm. That
is r, - r = 1 cm. In order to achieve this, choose a magnetic field with a
magnitude in Tesla (maximum magnetic field you can obtain from a
conventional magnet is around 2.5 T so be far away from this value) and
choose the direction also. Then determine the velocity of isotope you need.
Last calculate radius r of a Pbz04 smallest isotope.
Expert Solution
Step 1

Step 2
Step by step
Solved in 4 steps with 4 images
