
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
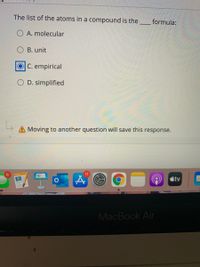
Transcribed Image Text:The list of the atoms in a compound is the.
formula:
O A. molecular
B. unit
C. empirical
O D. simplified
Moving to another question will save this response.
6.
11
étv
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many atoms of C are there in 25.0 g of pentane? (Atoms-molecules-moles-mass conversions; organic chem) a. 3.01×1024 b. 1.74 c. 1.04×1024 d. 1.25×1023arrow_forward. How many moles are in 18.5 g lead? Select one: a. 0.0893 mol b. 0.894 mol c. none of these d. 2.36 mol e. 2.35 mol f. 4.62 mol g. 4.63 molarrow_forwardHow many formula units up 20.0g of magnesium chloridearrow_forward
- Think about how to determine the molar mass of NH₂OH. In that amount of ammonium hydroxide, how many moles would you have? In other words, the molar mass of any compound is equal to A. 2 moles B. 1 mole C. 4 moles D. 0.5 molesarrow_forwardDirections. Calculate for the empirical formula of a given compound given their percentage composition and vice versa. (Note: You may use another sheet of paper to show your solution) 1. An analytical chemist determines that a compound of phosphorus and oxygen contains 43.64% P and 56.36% O. Find the empirical formula of the compound. 2. Find the empirical formula of a compound that contains 42.05% of Nitrogen and 95.95% of Oxygen.arrow_forwardone mole of aluminum contains how many atoms of aluminumarrow_forward
- An organic compound is known to contain only carbon, hydrogen, and oxygen. The compound contains, by mass, 39.1% of carbon and 8.7% of hydrogen. The number of carbon atoms in the empirical formula isSelect one: A. 2 B. 3 C. 4arrow_forwardCalculate the number of moles for 425g of KOH. A. 7.59 mol B. 16.5 mol C. 4.65 mol D. 1.25 molarrow_forward3. Aluminum oxide has the empirical formula of AlsOy a. Calculate the % aluminum in Al,O, using the chemical formula. b. Calculate the % error for the percent aluminum calculated in question 2. COPYRIGHT 2013 Cengage Learningarrow_forward
- Which of the following molecules containing nitrogen and oxygen has identical empirical and molecular formulas? A. NO B. NO2 C. N2O D.N2O4arrow_forwardHow do you Convert the mass of the following compounds to moles? a. 55 grams NaCl ________________ b. 47 grams of SbHFBr _________________________arrow_forwardHow many moles are in 18.5 g helium? Select one: a. none of these b. 4.62 mol c. 0.894 mol d. 4.63 mol e. 2.36 mol f. 0.0893 mol g. 2.35 molarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY