
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
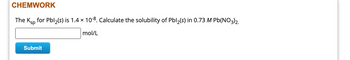
Transcribed Image Text:CHEMWORK
The Ksp for Pbl₂(s) is 1.4 × 10-8. Calculate the solubility of Pbl₂(s) in 0.73 M Pb(NO3)2.
mol/L
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Solubility Product Constant for magnesium fluoride is 6.4 × 10-9 Solid ammonium fluoride is slowly added to 75.0 mL of a 0.0304 M magnesium nitrate solution. The concentration of fluoride ion required to just initiate precipitation is M.arrow_forwardWhat is the molar solubility of Ca(OH)2 in a 0.340 M NaOH solution? The Ksp of Ca(OH)2 is 6.5 x 10^-6arrow_forwardDetermine the molar solubility for Cd₃(PO₄)₂ (Ksp = 2.5 × 10⁻³³) in a solution that already contains 0.500 M Na₃PO₄. (ICE table)arrow_forward
- What is the solubility of PbF₂ in a solution that contains 0.0500 M F⁻ ions? (Ksp of PbF₂ is 3.60 × 10⁻⁸)arrow_forwardAn analytical chemist has been given the task of precipitating lead cation (Pb2+) out of solution that is 0.424 M in Pb2+ . At her disposal is a large bottle of solid sodium iodide (NaI). What must the concentration of iodide be for precipitation to begin? (The Ksp of lead iodide is 9.8 x 10-9).arrow_forwardCalculate the solubility of PbI2 solubility in a 0.20 M Pb(NO3)2 solution. Ksp 1.4 x 10^-8.arrow_forward
- What is the molar solubility of strontium carbonate in a solution that contains 0.19-M SrCl2?Ksp(SrCO3) = 9.3 × 10-10 mol/L=arrow_forwardThe molar solubility of PbBr2 at 25 °C is 1.0 * 10-2 mol/L. Calculate Ksparrow_forwardWhat is the solubility of Mg(OH)₂ at a pH of 12.70? (Ksp Mg(OH)₂ is 1.6 × 10⁻¹³) Consider the titration of 30.0 mL of 0.275 M weak base B (Kb = 1.3 x 10⁻¹⁰) with 0.150 M HI. What is the pH of the solution before any acid has been added? Consider the titration of 30.0 mL of 0.275 M weak base B (Kb = 1.3 x 10⁻¹⁰) with 0.150 M HI. What would be the pH of the solution after the addition of 20.0 mL of HI?arrow_forward
- What is the solubility of magnesium hydroxide in a solution buffered at pH 8.95? Kp (Mg(OH)2) =1.8 x 10-11 Solubility g/Larrow_forwardDetermine the molar solubility of MF2 in a solution containing 0.0952 M KF. Ksp MF2 = 1.71 × 10–6 0.0952 M 7.52 × 10–3 M 1.80 × 10–5 M 1.89 × 10–4 M 9.25 × 10–4 Marrow_forwardA chemistry graduate student is given 500. mL of a 0.70M propanoic acid (HC₂H,CO₂) solution. Propanoic acid is a weak acid with K=1.3 × 10. What mass of NaC₂H,CO₂ should the student dissolve in the HC₂H5CO₂ solution to turn it into a buffer with pH = 4.52? You may assume that the volume of the solution doesn't change when the NaC₂H,CO₂ is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. 0 Explanation 83°F Mostly sunny A Check F4 $ X C % Ś F6 4 Q Search F7 & FB alc H F9 F10 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use F11 F12 PrtSc Insert Privacy Center | Accessi 4) Delete Backspacearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY