
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the IUPAC name for the following
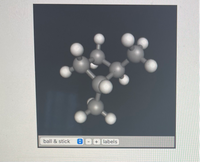
Transcribed Image Text:**Molecular Model: Ball and Stick Representation**
This image demonstrates a three-dimensional molecular structure using the ball-and-stick model representation commonly used in chemistry to visualize the structure of molecules.
**Description:**
- **Atoms:** The spheres (balls) in the model represent atoms. The color and size of the spheres typically correlate with different types of atoms (for example, carbon atoms are usually depicted as grey spheres, hydrogen atoms as white spheres, oxygen atoms as red spheres, etc.). However, in this image, all atoms appear in grayscale, making it less specific.
- **Bonds:** The rods (sticks) connecting the spheres represent the chemical bonds between atoms. The length and angles of these sticks follow the molecular geometry of the substance depicted.
**This Model:**
The molecule shown likely involves carbon and hydrogen atoms, considering the typical gray and white ball-and-stick colors associated. The arrangement suggests a relatively complex organic molecule, possibly an isomer of an alkane or a molecule with a significant number of carbon atoms.
**Functionalities in the Image:**
- **Ball & Stick:** A menu option indicating the user can change the type of model representation (ball-and-stick in this case).
- **Zoom Options:** Indicated by the plus ("+") and minus ("-") signs, these options allow for zooming in and out on the model to analyze different structural features.
- **Labels:** A button that could toggle labels on or off, providing additional information about each atom in the model such as element symbols or atomic numbers.
**Educational Context:**
This model is especially useful for students and educators in visualizing and understanding molecular structures, bond formations, and spatial arrangements in organic and inorganic chemistry. It provides a tangible way to comprehend abstract chemical formulas and reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please explain why 4-ethyl-5,5-dimethylpentane is the wrong IUPAC name?arrow_forwardprovide the IUPAC name of the product when 2-butanol undergoes oxidationarrow_forwardWhat is the IUPAC name for this molecule: 3-ethyl-2-methylpentane 3-ethyl-4-methylpentane 3-isopropylpentane 2-methyl-3-ethylpentanearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY