Question
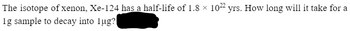
Transcribed Image Text:The isotope of xenon, Xe-124 has a half-life of 1.8 × 1022 yrs. How long will it take for a
1g sample to decay into lug?
Expert Solution
arrow_forward
Concept and Principle:
- Radioactive decay takes place when an unstable nucleus becomes stable. The instability arises due to a mismatch between the number of neutrons and protons. Only a few configurations of neutrons and protons are stable.
- The radioactive decay will be exponential in nature and is given by,
Here N0 is the original number of radioactive nuclei present, t is the time, and t1/2 is the half-life of the radioactive sample. This is known as the exponential law of radioactive decay.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Recently unearthed discoveries in North and South American archaeological sites showthat charcoal from ancient fires gave a 14C decay rate of 0.4 dpm/g. The 14C/12C ratio in livingorganisms is 1.3 x 10-12 with a decay rate of 15 dpm/g. What is the approximate age of thesample? The half-life of 14C is 5730 yearsarrow_forwardCarbon is one of the fundamental elements in everything that's alive on Earth. The three naturally occuring isotopes of carbon are 12C (mass fraction = 0.99), 1³C (mass fraction = 0.01) and radioactive 14C. The relative abundance or mass fraction of 14C is about 10-12 (or 1 in 1012 atoms). This radiocarbon originates from cosmic rays interacting with nitrogen atoms in the atmosphere and has a half-life time of 5730 years. Since it's half-life time is a lot higher than the time the atoms need to go through the carbon cycle, all living biomass at the Earth's surface has the same abundance of 14C as in the atmosphere. After a plant or animal has deceased the amount of 14C atoms will decay exponentially according to the following formula: = mo · 2-t/tı/2 with mo as the mass fraction at t = 0 and t1/2 as the half-life time. Since we know the abundance of 14C in the atmosphere, we can use this equation to estimate the time of death of an organism. This method is known as carbon dating.…arrow_forwardTritium, or hydrogen-3, is formed in the upper atmosphere by cosmic rays, similar to the formation of carbon-14. Tritium has been used to determine the age of wines. A certain wine that has been aged in a bottle has a tritium content only 71% of that in a similar wine of the same mass that has just been bottled. How long has the aged wine been in the bottle? The half-life of tritium is 12.3 y.arrow_forward
- The half life for the decay of carbon-14 is 5.73*10^3 years. Suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 6.2*10^2 Bq. The activity in a similar-sized sample of fresh wood is measured to be 1.1*10^3 Bq. Calculate the age of the artifact. Round your answer to 2 significant digits.arrow_forwardPlutonium-238 (238Pu) is a radioactive isotope with a half-life of 87.7 years. If a sample of 238Pu currently has an activity of 7000 decays/s, what was the activity 438.5 years (5 half-lives) ago?(a) 1.12×105 decays/s(b) 4.48×105 decays/s(c) 2.24×105 decays/s(d) 2.19×102 decays/s(e) 3.50×104 decays/sarrow_forwardA radioactive substance decays from 60mg to 12.6mg in 20 years according to the exponential decay model y=ae-bx, where a is the initial amount and y is the amount remaining after x years. a) find the b -value b) Use the exact b-value to write the exponential decay model for this substance with initial amount 60 mg, then use that model to find the half-life.arrow_forward
- The half-life for the decay of carbon-14 is 5.73*10^3 years. Suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 6.1*10^3 Bq. The activity in a similar-sized sample of fresh wood is measured to be 6.9*10^3 Bq. Calculate the age of the artifact. Round your answer to 2 significant digits.arrow_forwardA radioactive sample was measured 36.1 years ago. It had an activity of 99075.9 Bq. Today, its activity is 7.1 Bq. What is the decay constant (in years-1) of this substance?arrow_forwardA radioactive material produces 4.386E+3 decays per minute at one time, and 7.57 h later produces 679. decays per minute. What is its half-life, in hours?arrow_forward
- Iodine-125 (¹251) is used to treat, among other things, brain tumors and prostate cancer. It decays by gamma decay with a half-life of 54.90 days. Patients who fly soon after receiving ¹25I implants are given medical statements from the hospital verifying such treatment because their radiation could set off radiation detectors at airports. If the initial decay rate (or activity) was 522 µCi, what will the rate be after 377.0 days? decay rate: JCi How many months after the treatment will the decay rate be reduced to 16.5% of the original value? Assume months of 30 days. months:arrow_forwardA particular rock is thought to be 234 million years old. If it contains 4.84 mg of 238U, how much 206 Pb should it contain? Assume 238U decays directly to 206PB with a half-life of 4.47 × 109 y.arrow_forwardConsider a small pot with a copper base. The base has a thickness of 2.0 mm and a diameter of 15 cm. Water in this pot is boiling at 100 °C. Heat transfer rate is estimated at 250,000 J/s. Assume that heat enters the water only from the bottom of the pot through the copper base. Find the temperature of the heating element on which the copper bottom rests. 200 720arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios