
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
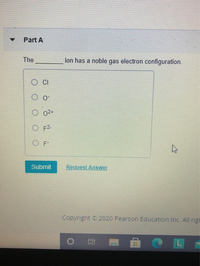
Transcribed Image Text:Part A
The
ion has a noble gas electron configuration.
O o
O 02+
O F2-
Submit
Request Answer
Copyright 2020 Pearson Education Inc. All righ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which atom will gain electrons during ionic bonding? A Mg B K C F D Liarrow_forwardA monatomic ion with a charge of -2 has an electronic configuration of 1s22s22p63s23p64s23d104p65s24d105p6.This ion is a(n) _______cation anion.What is the chemical symbol of the noble gas this ion is isoelectronic with? Xe.What is the formula of the ion? Ba2+.arrow_forwardAn atomic anion with a charge of -3 has the following electron configuration: [Ne] 3s 3p6 What is the chemical symbol for the ion? How many electrons does the ion have? How many 3p electrons are in the ion? П X Śarrow_forward
- Is K2S ionic and held together by opposite charge or shared electrons inarrow_forwardArrange the single covalent bonds within each set in order of INCREASING polarity. If: • most polar is b • medium polarity is c • least polar is a this leads to the following input: acb (To answer this question you may consult the table of electronegativity values in the table below.) 1A 2A Li Be 1.0 1.5 Na Mg 0.9 1.2 3B 4B 5B 6B 7B K Ca Sc Ti V 0.8 1.0 1.3 1.5 Rb Sr 0.8 1.0 1.2 Y Zr 1.4 <1.0 1.0-1.4 Cr Mn 1.5 1.6 1.6 Nb Mo 1.6 1.8 Cs Ba La Hf Ta 0.7 0.9 1.1 W 1.3 1.5 1.7 1.5-1.9 2.0-2.4 Fe 1.8 H 2.1 Re Os 1.9 2.2 8B Co Ni 1.8 1.8 1B 2B Cu Zn 1.9 1.6 Ir Pt Au 2.2 2.2 2.4 2.5-2.9 3.0-4.0 Te Ru Rh Pd Ag Cd In 1.9 2.2 2.2 2.2 1.9 1.7 1.7 3 A 4A 5A 6A 7A B N 0 F C 2.5 3.0 3.5 2.0 4.0 Hg 1.9 Al 1.5 Si P S CI 1.8 2.1 2.5 3.0 Ga Ge 1.6 As Se 1.8 2.0 2.4 Br 2.8 Sn Sb Te I 1.9 2.1 2.5 1.8 TI Pb Bi Po 1.8 1.8 At 1.9 2.0 2.2arrow_forwardGive the VSEPR and molecular shape for each elementarrow_forward
- How many valence electrons does phosphorus contain? O 10 O 15 O 5 3 2arrow_forwardWhich of the following answer choices explains how Lewis-Dot structures are used in Chemistry? O Lewis-Dot Structures are used to illustrate the valence electrons of an atom. Valence electrons are represented as dots around the element symbol of the atom. O Lewis-Dot Structures are used to illustrate the core electrons of an atom. Core electrons are represented as dots around the element symbol of the atom. O Lewis-Dot Structures are used to illustrate the protons of an atom. Protons are represented as dots around the element symbol of the atom. O Lewis-Dot Structures are used to illustrate the neutrons of an isotope. Neutrons are represented as dots around the element symbol of the atom.arrow_forwardStarting with 1s2draw out the complete electron configuration for the most stable ion of selenium. Explain how you used the Octet Rule to determine your answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY