
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The initial concentration of the reactant of a first order reaction is 8.46 M. If it takes 3.7 min for the remaining concentration to reach 0.46 M, what is the half-life of the reaction in units of s?
Report your answer to one decimal place.
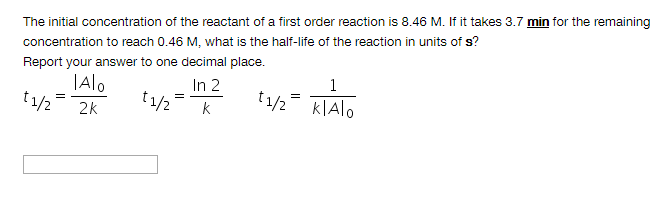
Transcribed Image Text:The initial concentration of the reactant of a first order reaction is 8.46 M. If it takes 3.7 min for the remaining
concentration to reach 0.46 M, what is the half-life of the reaction in units of s?
Report your answer to one decimal place.
t1/2
TAlo
In 2
t1/2
t1/2= KIAIO
2k
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the rate constant of a first-order reaction when 20.0% of a reactant remains after 78.0 s?arrow_forwardChoose all of the following that are true. Raising the temperature of a reaction will have no effect on the reaction rate. The units of the rate constant are independent of the overall reaction order. For a zero order reactant, tripling the concentration will have no effect on the rate. The plot of ln[A] vs time results in a straight line when a reaction is second order. Rates of reaction can be positive or negative. Activation energy is the maximum energy needed to initiate a chemical reaction.arrow_forwardAt a certain temperature this reaction follows first-order kinetics with a rate constant of 0.32 s : 2NH3(g) → N₂(g) + 3H₂(g) Suppose a vessel contains NH3 at a concentration of 0.540M. Calculate how long it takes for the concentration of NH3 to decrease to 5.0% of its initial value. You may assume no other reaction is important. Round your answer to 2 significant digits. 0x10 Xarrow_forward
- The generic reaction shown below follows second-order kinetics. 3 A + 2 B → products The half-life of A is 65.0 seconds when the initial concentration of A is 0.858 M. Determine the value of the rate constant for this reaction. Round your answer to 3 significant figures. You do not need to enter a unit.arrow_forwardWhich of the steps described here prevents the contamination of just-washed hands? use of a paper towel to turn off a faucet O lathering under running water for 30 seconds wetting the hands before applying soap use of hot water.arrow_forwardAge of a tree/fossils can be measured by looking at the remaining Carbon-14 in a sample. C-14 has a half-life of 5720 years and this is a first-order reaction. If a piece of wood has converted 88.5% of the carbon-14, then how old is it?arrow_forward
- A first-order reaction has a half-life of 25.5 s. How long does it take for the concentration of the reactant in the reaction to fall to one-sixteenth of its initial value? Express the time to three significant figures and include the appropriate units. HẢ ? t = Value Unitsarrow_forwardAt a certain temperature this reaction follows first-order kinetics with a rate constant of 0.911 s NH,OH (aq) → NH3 (aq) +H,0 (aq) Suppose a vessel contains NH,OH at a concentration of 0.360 M. Calculate the concentration of NH,OH in the vessel 0.680 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. IMarrow_forwardThe half-life of a pesticide determines its persistence in the environment. A common pesticide degrades in a first-order process with a half-life of 6 days. What fraction (in decimal notation) of the pesticide remains in the environment after 20 days? Enter to 4 decimal places.arrow_forward
- A first-order reaction has a half-life of 15.5 s . How long does it take for the concentration of the reactant in the reaction to fall to one-sixteenth of its initial value? Express the time to three significant figures and include the appropriate units.arrow_forwardA sample of 0.900 mol N2O is placed in a sealed container, where it decomposes irreversibly to N2 and O2 in a first-order reaction. After 42.0 min, 0.640 mol N2O remains. How long will it take for the reaction to be 90.0% complete?arrow_forwardThe initial concentration of a reactant in a first order reaction is 0.860 M. What will be its concentration after 1 half-lives have passed?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY