
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
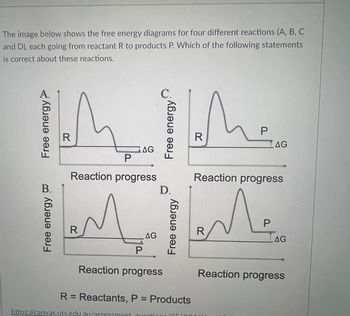
Transcribed Image Text:The image below shows the free energy diagrams for four different reactions (A, B, C
and D), each going from reactant R to products P. Which of the following statements
is correct about these reactions.
R
P
Reaction progress
ملك
R
AG
P
https://canvas.uts.edu.au/assessment
AG
D.
Reaction progress
R: Reactants, P = Products
questions (35(
R
P
R
LAG
Reaction progress
Me
P
LAG
Reaction progress

Transcribed Image Text:Reactions A and B are both spontaneous
Reactions A and C are both exergonic
Reactions A and B are both endergonic
Reactions C and D are both energetically favourable
Reactions C and D are both spontaneous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2×10-4 at a certain temperature. If a solid sample of NH4SH decomposes, what is the equilibrium concentration of NH3 be?arrow_forwardNH3(g) + H2O(l) → NH2 + + H3O+ t The equilibrium constant is 1 x 10 -34. Is this reaction likely to take place? Why? Choose the best answer below. a. Yes, because the equilibrium constant is very small and equilibrium will lie far to the left. b. Yes, because the equilibrium constant is very small and equilibrium will lie far to the right. c. No, because the equilibrium constant is very small and equilibrium will lie far to the right. ☐ d. No, because the equilibrium constant is very small and equilibrium will lie far to the left. Click Submit to complete this assessment.arrow_forwardConsider the exothermic reaction: A (g) + B (s) → 2X (g) @ 25 °C a. Propose a change in VOLUME so that the equilibrium position shifts towards the REACTANTS. b. Propose a change in TEMPERATURE so that the equilibrium position shifts towards the PRODUCTS. c. Propose a change in [A] so that the equilibrium position shifts towards the PRODUCTS.arrow_forward
- How do you solve HWK1?arrow_forwardYou are given the following: H2 (g) + Br2 (g) 2 HBr (g) Kp = 7.9 x 1011 H2 (g) 2 H (g) Kp = 4.8 x 10-41 Br2 (g) 2 Br (g) Kp = 2.2 x 10-15 What is Kp for the reaction shown below? H (g) + Br (g) HBr (g) Kp = ?arrow_forwardConsider the following equilibrium reaction: 2 NO (g) + O2 (g) ⇋⇋ 2 NO2 (g) What is the Kc expression? Select one: a. Kc=[NO2][NO][O2]Kc=[NO2][NO][O2] b. Kc=[NO2]2[NO]2+[O2]Kc=[NO2]2[NO]2+[O2] c. Kc=[NO]2[O2][NO2]2Kc=[NO]2[O2][NO2]2 d. Kc=2∗[NO2]2∗[NO][O2]Kc=2∗[NO2]2∗[NO][O2] e. Kc=[NO2]2[NO]2[O2]arrow_forward
- Good hand written explanation Asap thanks Consider the reaction: AC (g) + 2 B (g) ↔ AB2 (g) + C (g) ΔH = -224.5 kJ The system is sitting at equilibrium. Describe what will happen when each of the following stresses are applied. The reaction will shift to the right.The reaction will shift to the left.The reaction will not be affected by this. B is selectively removed from the reaction vessel. The reaction will shift to the right.The reaction will shift to the left.The reaction will not be affected by this. Additional C is added to the reaction vessel. The reaction will shift to the right.The reaction will shift to the left.The reaction will not be affected by this. The temperature of the reaction vessle was raised.arrow_forwardGiven this reaction at equilibrium, C(s) + CO2(g) D 2CO(g) (∆H° = 119kJ), explain the changes that would occur when the following stresses are applied: a.CO is removed. b.Heat is removed. c.CO2 is added.arrow_forwardPlease show all your work please, thank you! Sorry, this question has many parts. The reaction O3(g)+O(g)→2O2(g), occurs via the following mechanism:Step 1: Cl(g)+ O3(g)→ClO(g)+O2(g); Ea=85 kJ/molStep 2: ClO(g)+ O(g)⇌Cl(g)+O2(g); Ea=10 kJ/mol Using the proposed mechanism, derive a rate law. *Hint: use the activation energies to determine the slower step. Show all of your work.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY