
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
I am struggling with this question
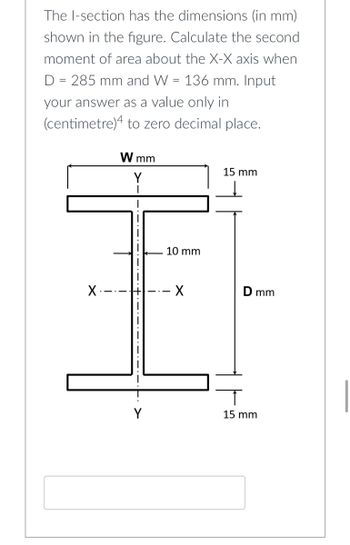
Transcribed Image Text:The I-section has the dimensions (in mm)
shown in the figure. Calculate the second
moment of area about the X-X axis when
D = 285 mm and W = 136 mm. Input
your answer as a value only in
(centimetre)4 to zero decimal place.
W mm
15 mm
X.
Y
10 mm
X
D mm
15 mm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- That is the answer I already have, I am lookinf for the answers to the blanks in the imagearrow_forwardAn electric hot water heater consumes 3.1 kilowatts of electricity and converts it to heat. How long will it take the water heater to heat a 67 gallon tank of water from 10 degrees Celsius to 50 degrees Celsius? (1 kilogram of water is 0.37 gallons, 1 Calorie = 4200 J). It may be helpful to refer back to the weekly handout for guidance on this problem. Your final answer should be in minutes (rounded to the nearest 10 minutes).arrow_forwardOn F2, how to identify the if we need to multiply it by "(4/5) and (3/5) slopes"?arrow_forward
- can you provide an answer easie to read. the answer is put into sentences like fcv=mgcos 6arrow_forwardHello. Can you please help answer the question shown in the photo? It is a 3-part question which I have attempted many times. I was able to calculate the correct answer for part 2, but part 1 and part 3 still says I am incorrect. Please show how to properly solve the problem. The topic is heat transfer. Thank you.arrow_forwardi) Find the property that provides the change in size of the material, if the material is subjected to temperature change and explain that property with unit. ii) Water of 1.6 liters is heated to a maximum temperature of 116 °C from room temperature to prepare Arabic coffee for a family using the following data. -- (2.5 Marks) - Heat energy of 623 kJ is supplied to the water - Specific heat capacity of water is 4.2 kJ/kg K - Density of water as 1000.5 kg/m3 Determine the room temperature. a) Mass of water (in kg) b) Room temperature (in °Carrow_forward
- 1. An ingot of mass 1,148 kg contains copper, tin, and lead in the ratio 9:4:1. Calculate the value of the ingot if the price per tonne of the metals is copper: $ 1,250, lead: $ 680, tin: $ 8,600.arrow_forwardA gas filled in a piston-cylinder arrangement as shown in Figure 1 has a volume of 0.09 m³ at its initial equilibrium state. The gas is slowly heated through the bottom of the cylinder that allowed for the gas to expand at constant-pressure of 2095 kPa to a volume of 1.23 m°. The change in internal energy of the gas is 0.24 kJ. Ignoring the friction between the piston and the cylinder and assuming the atmospheric pressure any value from 90 to 110 kPa, of (a) Calculate the mass of the piston if the area of the piston is 0.047 m2 (b) For the gas as a system, evaluate work and heat transfer, each in kJ (c) For the piston as a system, evaluate work and change in potential energy, each in kJ Piston Gasarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY