
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Answer = kJ/K
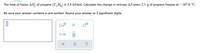
Transcribed Image Text:The heat of fusion AH, of propane (C₂H) is 3.5 kJ/mol. Calculate the change in entropy AS when 2.1 g of propane freezes at -187.8 °C.
Be sure your answer contains a unit symbol. Round your answer to 2 significant digits.
Ú
н
x10
ロ・ロ
X
Ś
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer all parts of the question ❤️ kinetic energy answers 1 (lowest) - 4 (highestarrow_forwardQ1: The heat of combustion of Cuhio butane is 2878-5 - when Co2 is given and the liquid water chemistry material rning point is 58.5 26- Live what is given CO2 and boil water that is very hot chemistry materialarrow_forwardThe cooling system in an automobile holds 11.1 x 103 g of ethylene glycol antifreeze. How much energy is absorbed from the engine when the temperature of the ethylene glycol goes from 20.0°C to 100.0°C? The specific heat capacity of ethylene glycol is 2.42 J/(g-*C). Round your response to 3 significant digits. Type your answer...arrow_forward
- Select all the expressions equal to (p.a) = (x c) Ор Op⋅a÷x.c pa q Op a÷ (x c) 1 C.C (p.a) x c IC □ (p.a). Op a I C p.a T I. Carrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 7.80 kg of water at 32.2 °C. During the reaction 51.4 kJ of heat flows out of the flask and into the bath. - 1 Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18 J'g ''K'. -1 Round your answer to 3 significant digits. x10arrow_forward2,4-Dichlorophenoxyacetic acid ("2,4-D") is a widely used herbicide with the molecular formula C8H6Cl2O3C8H6Cl2O3. The pKapKa of 2,4-D is 2.73. A certain water-based herbicide product contains 130 gL−1gL−1 of 2,4-D. What is the pHpH of the 2,4-D solution?arrow_forward
- ▾ Part A DA chemical reaction does not occur for this question When ethanol (C₂HO(aq)) is consumed, it reacts with oxygen gas (O₂) in the body to produce gaseous carbon dioxide and liquid water. Enter the balanced chemical equation for the reaction Express your answer as a chemical equation including phases. | ΑΣΦ Submit Previous Answers Request Answer x Incorrect: Try Again; 6 attempts remaining SARASER E Provide Feedback MAIKLIENT Next >arrow_forward18arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY