
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
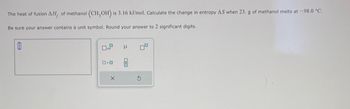
Transcribed Image Text:The heat of fusion AH, of methanol (CH,OH) is 3.16 kJ/mol. Calculate the change in entropy AS when 23. g of methanol melts at -98.0 °C.
Be sure your answer contains a unit symbol. Round your answer to 2 significant digits.
0
0
X
H
010
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the following experiment, solid CaCl2 (M = 110.98 g/mol) is dissolved in water:CaCl2(s) → Ca2+(aq) + 2Cl-(aq) ΔsolnH = ? kJ mol−1A 4.00 g sample of CaCl2 is added to a coffee-cup calorimeter containing 125 g of H2O. The initial temperature of water in the calorimeter is 22.0°C and the final temperature is 27.5°C. What is the enthalpy change for the dissolution reaction (ΔsolnH) in kJ per mole of CaCl2? Assume the specific heat capacity of the solution is the same as water (Cs=4.18 J g-1 °C-1) and that no heat is lost to the calorimeter.arrow_forward3 If we measure the heat change (q) of everything in a system except the calorimeter, we can use the first law of thermodynamics (∆Euniverse = 0) to calculate the heat change of the calorimeter (qcal). In this reaction, we combine 5.75 g of 67.4 ºC water with 6.67 g of 19 ºC water in a calorimeter. Since some of the heat is absorbed by the calorimeter, the final temperature of the water is 25.4ºC. Since the hot and cold water are combined, the final temp of the water is the final temp for the hot water and the cold water. Calculate the heat change of the calorimeter in J. Remember that all of the heat changes must add up to zero so you can calculate the heat change of the hot water (qhotwater) and the heat change of the cold water (qcoldwater) using for each where Cs = 4.186 J/gºC. The first law of thermodynamics tells us that all of these changes sum up to zero so Enter your answer to one decimal place (tenths)arrow_forwardFind the ΔH (in kilojoules) for the reaction below, given the following reactions and subsequent ΔH values: (Write your answer in 1 decimal place without the unit). N2(g) + 2O2(g) → 2NO 2(g) N2(g) + 3H2(g) → 2NH3(g) ΔH = -115 kJ 2NH3(g) + 4H2O(l) → 2NO2(g) + 7H2(g) ΔH = -142.5 kJ H2O(l) → H2(g) + 1/2O 2(g) ΔH = -43.7 kJarrow_forward
- Calcium acetate, Ca(CH3COO)2 has a molar mass of 158.17 g/mol. In a constant pressure calorimeter, 44.2 g of Ca(CH3COO)2 is dissolved in 861g of water at 25.00 °C. The heat produced by the solution was determined to be 15kJ. What is the final temperature of the solution? q= - msAT SH20 = 4.184 29.0 °C O 18.4 °C O 64.6 °C O 33.8 °C O 3.96 °C O 21.0 °Carrow_forwardFor the reactionCO(g) + Cl2(g) COCl2(g)G° = -62.9 kJ and S° = -137.3 J/K at 331 K and 1 atm.This reaction is (reactant, product) fill in the blank 1 favored under standard conditions at 331 K.The standard enthalpy change for the reaction of 2.37 moles of CO(g) at this temperature would be kJ.arrow_forward55. Does the standard enthalpy of formation of H2O(g) differ from ΔH° for the reaction 2H2(g)+O2(g)⟶2H2O(g)?arrow_forward
- Consider the reaction 2H2O(g) →2H2(g) + O2(g) ΔH = +483.60 kJ/mol at a certain temperature. If the increase in volume is 52.7 L against an external pressure of 1.00 atm, calculate ΔU for this reaction. (The conversion factor is 1 L · atm = 101.3 J.) put your answer in KJarrow_forwardIf a system has 5.00×102 kcal5.00×102 kcal of work done to it, and releases 5.00×102 kJ5.00×102 kJ of heat into its surroundings, what is the change in internal energy (Δ? or Δ?)(ΔE or ΔU) of the system?arrow_forwardWhat is the specific heat capacity of a substance of mass 159.5 g that increases in temperature from 22.2 °C to 25.2 °C after absorbing 1.15 kJ of energy in the form of heat? Express your answer in J g-1 °C-1. Do not try to identify the substance.arrow_forward
- Suppose 9.44 g of calcium chloride, CaCl2, (MM=111.0 g/mol) is added to 222. g water at 21.84oC in a styrofoam cup calorimeter, and the temperature rose to 29.76oC. Calculate a value for ΔH in the equation: CaCl2(s) + H2O(l) → Ca2+(aq) + 2Cl-(aq) ΔH = ??? kJ/mol. (Assume that the styrofoam cup does not absorb any heat nor allow any heat loss; assume the specific heat of the resulting solution is 3.88 J/(goC))arrow_forwardA calorimeter contains 16.0 mLmL of water at 12.5 ∘C∘C . When 1.40 gg of XX (a substance with a molar mass of 75.0 g/molg/mol ) is added, it dissolves via the reaction X(s)+H2O(l)→X(aq)X(s)+H2O(l)→X(aq) and the temperature of the solution increases to 25.0 ∘C∘C . Calculate the enthalpy change, ΔHΔ�, for this reaction per mole of XX. Assume that the specific heat of the resulting solution is equal to that of water [4.18 J/(g⋅∘C)J/(g⋅∘C)], that density of water is 1.00 g/mLg/mL, and that no heat is lost to the calorimeter itself, nor to the surroundings. Express the change in enthalpy in kilojoules per mole to three significant figures.arrow_forwardConsider the combustion of propane: C3H (9) + 50, (9)→ 3CO, (9) + 4H2 O(1) AH = -2221 kJ A balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 4.00 x 10° L to 4.50 x 10° L by the addition of 1.4 x 10 J energy as heat. Assume that all the heat comes from the combustion of propane. What mass of propane must be burned to furnish this amount of energy assuming the heat transfer process is 50.% efficient? Mass =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY