
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
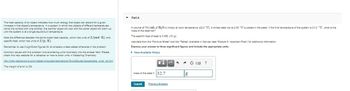
Transcribed Image Text:The heat capacity of an object indicates how much energy that object can absorb for a given
increase in that object's temperature. In a system in which two objects of different temperatures
come into contact with one another, the warmer object will cool and the cooler object will warm up
until the system is at a single equilibrium temperature.
Note the difference between the terms molar heat capacity, which has units of J/(mol-K). and
specific heat, which has units of J/(g-K).
Remember to use 3 significant figures for all answers unless stated otherwise in the problem.
Common issues with this problem involve entering units incorrectly into the answer field. Please
check this help website for a refresher on how to enter units in Mastering Chemistry:
http://help.pearsoncmg.com/mastering/student/standalone/TopicsStudent/acceptable units list.htm
The margin of error is 2%.
Part A
A volume of 75.0 mL of H₂O is initially at room temperature (22.0 °C). A chilled steel rod at 2.00 °C is placed in the water. If the final temperature of the system is 21.0 °C. what is the
mass of the steel bar?
The specific heat of steel is 0.452 J/(Kg).
Use data from the "Formula Sheet" and the "Tables" available in Canvas (see "Module 0: Important Files") for additional information.
Express your answer to three significant figures and include the appropriate units.
▸ View Available Hint(s)
mass of the steel = 32.7
Submit
Previous Answers
μA
1 →
g
(3 ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. A calorimeter contains 100.0 g of water at 39.8 °C. A 12.78 g object at 100.0 ºC is placed inside the calorimeter. When equilibrium has been reached, the new temperature of the water and metal object is 40.6 °C. What is the specific heat of the metal?arrow_forwardWhen a 8.00 g8.00 g sample of RbBrRbBr is dissolved in water in a calorimeter that has a total heat capacity of 3.03 kJ⋅K−1,3.03 kJ⋅K−1, the temperature decreases by 0.350 K.0.350 K. Calculate the molar heat of solution of RbBr.arrow_forwardThe temperature of a 15-g sample of lead metal increases from 22 °C to 37 °C upon the addition of 29.0 J of heat. The specific heat capacity of the lead is J/g-K.arrow_forward
- Copper has been used for thousands of years, either as a pure metal or in alloys. It is frequently used today in the production of wires and cables. Copper can be obtained through smelting or recycling. Determine the energy associated with each of these processes in order to recycle 1.40 mol Cu. The smelting of copper occurs by the balanced chemical equation: CuO(s) +CO(g) → Cu(s) +CO,(g) where AHtCuo is = - 155 kJ/mol. Assume the process of recycling copper is simplified to just the melting of the solid Cu starting at 25°C. The melting point of Cu is 1084.5°C with AH®fus = 13.0 kJ/mol and a molar heat capacity, CPCU = 24.5 J/mol:°C.arrow_forwardA gaseous system has 71 J of work done on it by surroundings and increases its internal energy by 69 J. Calculate the amount of heat associated with this scenario. 140 J of heat is released by the system. 2 J of heat is released by the system. The heat of the system remains constant. 2 J of heat is absorbed by the system. 140 J of heat is absorbed by the system.arrow_forwardA 1.75 g sample of zinc metal is reacted with 128 g of 1.10 M hydrochloric acid solution in a constant-pressure calorimeter. The resulting solution changes in temperature from 22.58 °C to 22.97 °C. The hydrochloric acid is in excess. Based on this information, and estimating the solution's heat capacity as 4.18 Jg1 °C, what is the amount of heat, in joules, transferred in this reaction?arrow_forward
- As a system increases in volume, it absorbs 55.0 J of energy in the form of heat from the surroundings. The piston is working against a pressure of 0.540 atm. The final volume of the system is 58.2 L. What was the initial volume of the system if the internal energy of the system decreased by 103.4 J?arrow_forwardIron has a specific heat capacity of 0.444J/g-C. 50.0 g of iron at 35C is added to 50.0 g of water with a specific heat capacity of 4.184 J/g-C. When the temperature of the resulting mixture stops changing, the temperature is closer to that of the original water than to the iron. True or false?arrow_forwardA particular container holds 3.77 mol of neon gas. The volume of this container can be altered by sliding a piston in or out. The volume is changed from 8.60 L to 12.20 L while at the same time the temperature is changed from 303 K to 345 K. The molar heat capacity, Cym, for neon is 12.47 J/(mol · K). Assume that this value will not change over the given temperature range. What is the change in entropy for the gas? AS = J/K TOOLS x10 MacBook Proarrow_forward
- Use the References to access important values if needed for this question. A student determines the heat of dissolution of solid potassium bromide using a coffee-cup calorimeter of negligible heat capacity. When 6.36 g of KBr(s) is dissolved in 115.00 g of water, the temperature of the solution drops from 25.00 to 22.71 °C. Based on the student's observation, calculate the enthalpy of dissolution of KBr(s) in kJ/mol. Assume the specific heat of the solution is 4.184 J/g°C. AHdissolution = kJ/mol Submit Answer 5 question attempts remainingarrow_forwardEnergy in the amount of 5.0 J is added to 0.50 g pieces of aluminum, copper, and magnesium that are initially kept at 22 °C. The molar heat capacities of these metals are 24.3 J/(mol·°C), 24.5 J/(mol·°C), and 24.9 J/(mol·°C), respectively. Which piece of metal will have the highest temperature and what is that temperature?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY