
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
2r
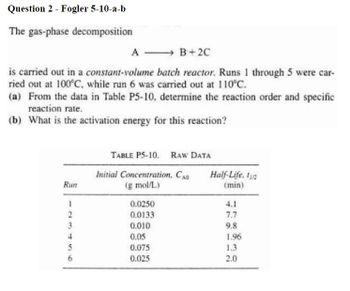
Transcribed Image Text:Question 2 - Fogler 5-10-a-b
The gas-phase decomposition
B+2C
is carried out in a constant-volume batch reactor. Runs 1 through 5 were car-
ried out at 100°C, while run 6 was carried out at 110°C.
(a) From the data in Table P5-10, determine the reaction order and specific
reaction rate.
(b) What is the activation energy for this reaction?
Run
1
2
3
4
5
56
6
A
TABLE P5-10. RAW DATA
Initial Concentration, CAO
(g mol/L)
0.0250
0.0133
0.010
0.05
0.075
0.025
Half-Life, t
(min)
7.7
9.8
1.96
1.3
2.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- 14-6² (4) Measurement the fuel efficiency for car FE at various speed as shown in table: v(mi/h): 5 25 15 35 75 45 55 65 FE 22 28 29-5 30 30 27 23 Curven't the data with a second-order polynomial. Use poly nemial to estimate the fuel efficiency at (Comi/h). Make aplot the points and the polynomial.arrow_forwardF1 F2 A2 P2 The following is known: Area, A1= 0.3 ft and A2= 7.6 A1- Assuming an incompressible fluid If a force F1 of 44.3 lbf / ft2 is applied at (1) then the resultant force, F2 in Ibf is equal to -- ?arrow_forwardA tire is inflated to a gage pressure of 35 psi at a temperature of 0ºF. Calculate the maximum temperature to which the tire may be heated without the gage pressure exceeding 50 psi. A. 110ºF B. 124ºF C. 139ºF D. 158ºFarrow_forward
- oner flat plate, I sothermal sur face (tenp is un ferm) Lauinar /turo / mixed Uoo L External flow Reca for flat ptale Sxios מ xף4סי5 Turonlent Find Rey 5.049 x 10> Reu 5.049 X 10 ニ く Lam turb 4/s 2 B.3 NuL= (o037 Re-A) Pr 2 Bo3 037 Re -A) Pr A=871 ニ Re< Rex Rey tuyb. 6904.3 16m ニ Lami now K JuL う fs t Nuv ニ %3Darrow_forward6.16. Using a colo below 01 moarrow_forwardConvert the unit of permeability in (m³ m³² s´¹) to the following units: cm³ cm-²h-¹ gal ft 2day-¹ L m² h-¹ L m² day-¹arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The