
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
2) The following substances were separated on a molecular exclusion (gel filtration) column. Estimate the molecular mass of the unknown
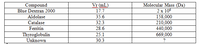
Transcribed Image Text:Vr (mL)
17.7
Compound
Molecular Mass (Da)
2 x 106
158,000
210,000
440,000
669,000
www
Blue Dextran 2000
Aldolase
35.6
Catalase
Ferritin
32.3
28.6
25.1
Thyroglobulin
Unknown
30.3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider doping of silicon with gallium.Assume that the diffusion coefficient of gallium in Si at 1100℃ is 7*10-13 cm2/s.calculate the concentration of Ga at depth of 2.0 micrometer if the surface concentration of Ga is 1023 atom s/cm3.The diffusion times are 1,2 and 3 hours.arrow_forwardFor the absorption of solute A through a falling film, its Peclet number, Npe is in proportion to Select one or more: A. The average velocity of the falling film. B. All of the given choices. C. Diffusivity (DAB) D. Thickness of the falling film E. The reciprocal of diffusivity (1/DaB) O OOSOD OOSOLarrow_forwardSketch how on silicon wafer NiCR is deposited? (Evaporation technique) Sketch how NiCR layer is coated with photoresist and exposed to UV light? Sketch how SU8 is deposited on NiCR layer and exposed to UV light?arrow_forward
- in a 2-hour carburizing treatment, what temperature is required to obtain a 0.5% concentration of carbon at a depth of 0.5 mm below the surface of steel with a carbon concentration of 0.2%?considers that the inert gas used is enriched with carbon atoms at a concentration of 1.10% and that iron has an fcc structurearrow_forward1. If the temperature decreases will the rate of diffusion will decrease or increase? 2. If the surface area for diffusion increases will the rate of diffusion increase or de- crease? 3. If equal amounts of Ar and He are being placed in a porous container and are al- lowed to escape, which substance will escape faster?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The