
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
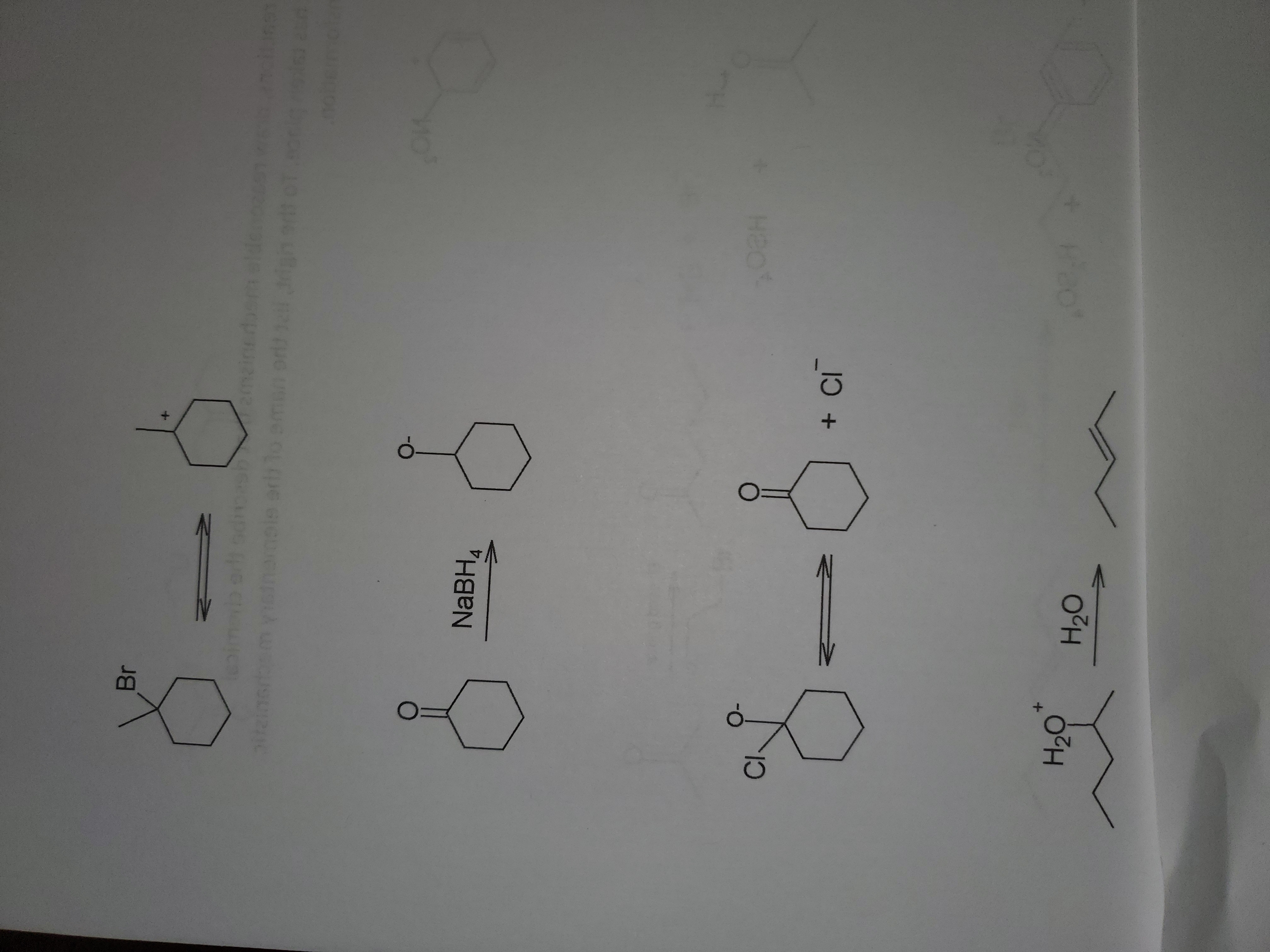
Given the following reactions, draw reasonable mechanisms that describe the chemical transformation that has taken place. To the right, list the name of the elementary mechanistic step used in the transformation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help provide an arrow pushing mechanism. The first step is to perform an electron transfer to form superoxide, but I am confused on where to go from there. isouramil e H₂O₂arrow_forwardPredict the major products of each of the following reactions. Draw the complete detailed mechanism that leads to the formation of these products. Letters D and Earrow_forwardDraw the SN2 mechanisms for reaction equation below. In addition, show the reaction energy diagram for formation of both products (please indicate AG, activation energies, transition states). Which reaction product will form faster? How does the reaction energy diagram for the SN2 reaction change when ethanol is used as a solvent instead of acetone. Please explain. 1 mmol )=S=( CH3 + 1.5 mmol Br solvent Brarrow_forward
- Draw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. Br O Substitution will not occur at a significant rate. : CN + X Click and drag to start drawing a structure.arrow_forwardFor which reaction mechanisms—SN1, SN2, E1, or E2—of thefollowing statement true? A statement may be true for one or moremechanisms. The mechanism is concertedarrow_forward2. The following chemical transformations involve multistep mechanisms. Please write a reasonable mechanism for each transformation and label each elementary step.arrow_forward
- Show and label the mechanism for the SN1 reaction using the generic chemical species and electron movement. Notice that the reaction does not include a negative charge on the nucleophile.arrow_forwardWrite the synthetic step that shows 2-phenyl-2tosylpropane reacting with NaCl to produce 2- chloro-2phenylpropane. Also draw the mechanism for this reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY