
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
7.46
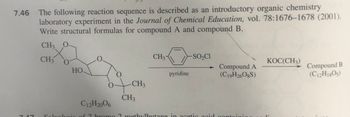
Transcribed Image Text:7.46
The following reaction sequence is described as an introductory organic chemistry
laboratory experiment in the Journal of Chemical Education, vol. 78:1676-1678 (2001).
Write structural formulas for compound A and compound B.
CH3 0
X
CH3
O
HO.
0
O
+
O
C12H2006
CH3
CH3
CH3
pyridine
SO₂C1
Soluolugin of ? bromo ? methylbutane in acetic acid
Compound A
(C19H26O8S)
ont
KOC(CH3)
Compound B
(C12H1805)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- consumptiOH TOr uinitiugen il kglS! (we will igui production in next activity) 2 QUESTION In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 592. liters per second of dinitrogen are consumed when the reaction is run at 249. °C and the dinitrogen is supplied at 0.27 atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits.arrow_forwardA local water retention pond was found to have elevated levels of mercury concentrated as 14 ng per liter. You would like to collect some mercury for your homemade perpetuum mobile machine. How many moles of mercury can you get you collect and process the entire volume of 1250 m3 of the water in the pond? 1.8 x 10-5 moles 4.4 x 10:5 moles 1.8 x 10-2 moles 8.7 x 108 moles 7.6 x 10-5 moles O 4.4 x 108 moles 8.7 x 105 molesarrow_forwardWhen the following equation is completed and balanced, what is/are the product(s)? MgO(s) + HCl(aq) --> ? (A) MgCl2(aq) + H2O(l) (B) MgOH2(aq) + Cl2(g) (C) MgOCl2(aq) + H2(g) (D) Mg(s) + Cl2(g) + H2O(l)arrow_forward
- Write the balanced NET ionic equation for the reaction when aqueous CS:PO4 and aqueous AGNO: are mixed in solution to form solid AgsPO4 and aqueous CSNO.. 5. 6 7. 8 6. 1 口 口。 口。L。 (s) (g) (aq) Cs Ag 白 百|2arrow_forwardD. E. F.arrow_forward(4.3: Similar to For Practice 4.4) Calcium reacts with oxygen to form calcium oxide: 2Ca(s) + O2(g) --> 2Cao(s) How many grams of oxygen gas are needed to react completely with 12.9 g Ca? O 10.3 g 18.0 g O 5.15 g O 2.57 garrow_forward
- A 4.72 g sample of CaClX H2O was heated in crucible to evaporate water. After heating and cooling the mass remaining in crucible is 3.56 g. answer the following questions. 1- The percent water in hydrated salt (%H2O) is (a) 24.58% (b) 55.74% (с) 66.17% (d) 100% 2- The value of (X) is (a) 1 (b) 6 (c) 5 (d) 2arrow_forwardDon’t understand 7arrow_forward6. 7. How many moles of hydronium ions are present in 56 mL of a 0.58 M H₂SO4 solution? (10²) 7. 8. How many moles of hydroxide ions are present in 3.5 grams of Calcium hydroxide, Ca(OH)₂? (2-step) (102) 8. 1600arrow_forward
- Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(IV) oxide. 4HCl(aq)+MnO2(s)⟶MnCl2(aq)+2H2O(l)+Cl2(g)4HCl(aq)+MnO2(s)⟶MnCl2(aq)+2H2O(l)+Cl2(g) A sample of 35.7 g MnO235.7 g MnO2 is added to a solution containing 45.7 g HCl.45.7 g HCl. What is the limiting reactant? HClHCl MnO2MnO2 What is the theoretical yield of Cl2?Cl2? theoretical yield: g Cl2g Cl2 If the yield of the reaction is 86.1%,86.1%, what is the actual yield of chlorine? actual yield:arrow_forward2. The density of acetic anhydride is 1.08 g/ml. What is the mass of 2.00 ml of acetic anhydride? How many moles of acetic anhydride are present in a 500.0 ml bottle of acetic anhydride?arrow_forwardWrite balanced chemical equations for the reactions below, for synthesizing other historical pigments: Solid mercury reacts with solid sulfur to form mercury (II) sulfide, which is called vermillion (a red pigment) 1. 2. Aqueous cadmium (II) chloride reacts with sodium sulfide to form aqueous sodium ions, aqueous chloride ions, and cadmium (II) sulfide, which is called cadmium yellow (a yellow pigment). aqueousarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY