
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![The following reaction is second-order in A and first-order in B:
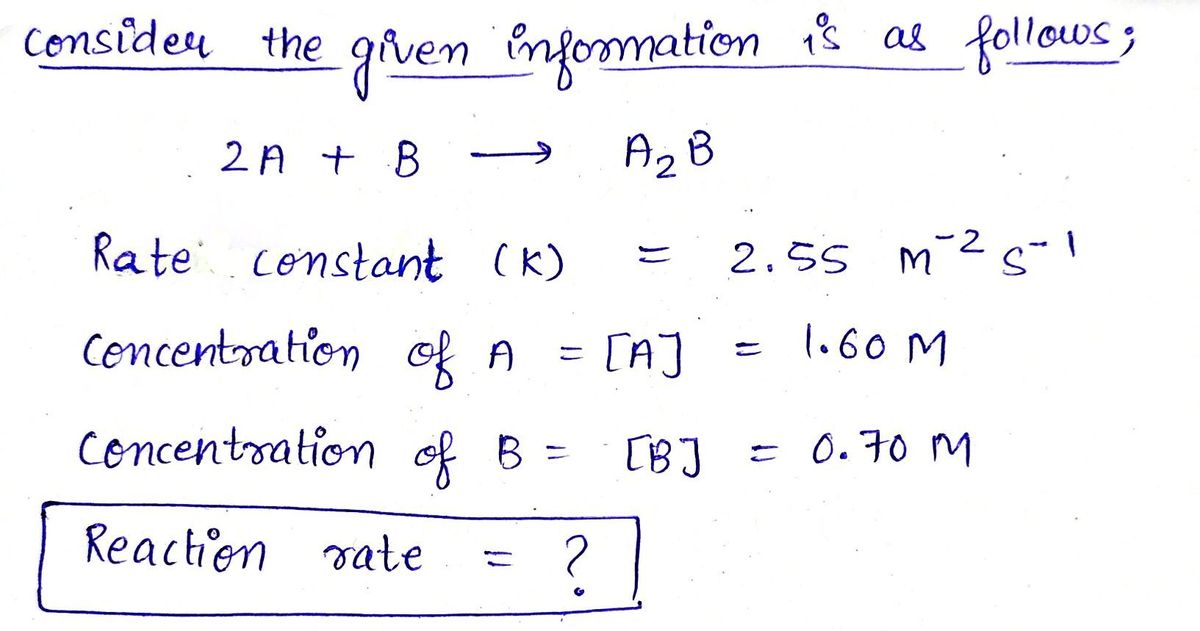
2 A+B → A₂B
What is the reaction rate if k = 2.55 M-2s¹ and [A] = 1.60 M [B] = 0.70 M?
Hint: Write down the correct differential rate law based on the order with respect to
A and B, then plug in the concentrations and k to calculate rate (R).
Your Answer:
Answer
units](https://content.bartleby.com/qna-images/question/d99dc5ee-0347-4b7c-8511-9a91331fbdb4/42b4f820-4d67-4764-8cc4-29d0fd5ea8ee/fc0lpab_thumbnail.jpeg)
Transcribed Image Text:The following reaction is second-order in A and first-order in B:
2 A+B → A₂B
What is the reaction rate if k = 2.55 M-2s¹ and [A] = 1.60 M [B] = 0.70 M?
Hint: Write down the correct differential rate law based on the order with respect to
A and B, then plug in the concentrations and k to calculate rate (R).
Your Answer:
Answer
units
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reduction of nitric oxide with hydrogen 2NO+2H₂ → N₂ + 2H₂O is second order in NO and first order in H₂. Complete the rate law for this reaction in the box below. Use the form k[A] [B]", where '1' is understood for m, n... (don't enter 1) and concentrations taken to the zero power do not appear. Rate = In an experiment to determine the rate law, the rate of the reaction was determined to be 0.0608 M-s¹, when [NO] = 0.498 M and [H₂] = 0.137 M. From this experiment, the rate constant is M-². s-¹.arrow_forwardAt a certain temperature this reaction follows second-order kinetics with a rate constant of 4.91 M s: 2HI (g) - H, (g) +I, (3) Suppose a vessel contains HI at a concentration of 1.33 M. Calculate the concentration of HI in the vessel 2.40 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. ?arrow_forwardThe gas phase reaction of hydrogen with iodine H₂+I2 → 2HI is first order in H₂ and first order in I2. Complete the rate law for this reaction in the box below. Use the form k[A] [B]", where '1' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not appear. Rate = In an experiment to determine the rate law, the rate constant was determined to be 2.03 x 10-18 M¹s¹. Using this value for the rate constant, the rate of the reaction when [H₂] = 0.0356 M and [1₂] = 0.0123 M would be M/s.arrow_forward
- The gas phase reaction of hydrogen with iodine H₂+ 12 → 2HI is first order in H₂ and first order in I2. Complete the rate law for this reaction in the box below. Use the form [A] [B]", where '1' is understood for m, n... (don't enter 1) and concentrations taken to the zero power do not appear. Rate In an experiment to determine the rate law, the rate constant was determined to be 2.39 x 10-18 M's. Using this value for the rate constant, the rate of the reaction when [H2]=0.0636 M and [12]=0.0154 M would be M/s. Submit Answer Retry Entire Group more group attempts remainingarrow_forwardPart B Consider the second-order reaction: 2HI(g)→H₂(g) + I₂(g) Use the simulation to find the initial concentration [HI], and the rate constant k for the reaction. What will be the concentration of HI after t = 5.44x10¹0 s ([HI]) for a reaction starting under the condition in the simulation? Express your answer in moles per liters to three significant figures. ► View Available Hint(s) for Part B for Part do for Part redo for Part B res@or Part B keyboard shortcuts for Part B help for Part B [HI] = 1.6810³ Submit Previous Answers X Incorrect; Try Again; 5 attempts remaining mol Larrow_forwardCan you explain the concept? How to apply the concept to this problem and other problems?arrow_forward
- Butadiene, C,H, (used to make synthetic rubber and latex paints) dimerize C3H12 with a rate law of rate = 0.014 L/mo[• s [CH]². If the remäining %3D concentration of CaH, is 0.028 M after 0.35 hours, what is the initial concentration of C4H, in molar? Report a numerical value with 3 decimal places, without units.arrow_forwardThe rate constant for the second order reaction 2 NO2 - 2 NO + O2 is 0.24 M's. If a 1.38 M sample of NO2 reacts for 47.7 seconds, what concentration of NO2 wil remain? Report your answer to two decimal places. |Al = - kt +|Alo InJAl = - kt + InlAlo TAI = kt + TAloarrow_forwardFe3+ (aq) + 7.00 SCN- (aq) → Fe(SCN)7.00 3+ (aq) 1.)Find the rate where SCN- is consumed, if Fe3+ is consumed at 1.00 M/min. 2.) Find the rate and the overall order of the rate lawarrow_forward
- Q2arrow_forwardBe sure to answer all parts. Consider the reaction A+BProducts From the following data obtained at a certain temperature, determine the order of the reaction. Enter the order with respect to A, the order with respect to B, and the overall reaction order. A. JA] (M) (BỊ (M) Rate (Mis) 1.50 1.50 3.20 x 10 1.50 2.50 3.20 x 10 Reaction 3.00 1.50 6.40 x 10arrow_forwardBe sure to answer all parts. The reaction of peroxydisulfate ion (S2O82−)with iodide ion (I− is S2O82−(aq) + 3I−(aq) → 2SO42−(aq) + I3−(aq) From the following data collected at a certain temperature, determine the rate law and calculate the rate constant. Experiment [S2O82−](M) [I−](M) Initial Rate [M/s] 1 0.0200 0.0520 4.80 × 10−4 2 0.0200 0.0260 2.40 × 10−4 3 0.0400 0.0260 4.80 × 10−4 (a) Which of the following equations represents the rate law for this reaction? A. rate = k[S2O82−][I−] C. rate = k[S2O82−][I−]2 B. rate = k[S2O82−]2[I−] D. rate = k[S2O82−]2[I−]2 (b) What is the rate constant for the reaction? k =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY