
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The following questions regard the chromatograms shown below:
Which column gives a greater selectivity factor for compound X and compound Y? Explain your answer.
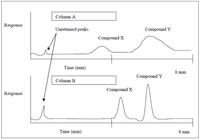
Transcribed Image Text:Column A
Response
Unretained peaks
Compound Y
Compound X
Time (min)
8 min.
Compound Y
Column B
Compound X
Response
Time (min)
8 min.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 7 In the Thermo Fisher application note on lon Chromatography (Lesson 4), a method is described on the second page. What mobile phase is used in the beginning of the method? O 10 mM KOH O 10 mM sodium carbonate O 10 ug/L bromate O 1000 ug/L NaF Question 8 The method discussed in the Thermo Fisher application note on lon Chromatography (Lesson 4) is an example of anion exchange chromatography. O True O Falsearrow_forwardQUESTION 5 In a chromatographic separation using a polar stationary phase and a mobile phase composed of 60% hexane and 40% ethyl acetate, two compounds, benzene (peak A) and toluene (peak B), are eluted. The width of peak A at its base is 1.2 min, the width of peak B at its base is 1.3 min, and the retention time of peak A is 5.2 min and peak B is 3.5 min. What is the resolution (Rs) between these two peaks and is it acceptable? R = 1.36. It is not an acceptable resolution. R = 0.74. It is an acceptable resolution. R = 1.36. It is an acceptable resolution. R = 0.74. It is not an acceptable resolution.arrow_forwardI would need help getting a example/drawing and explanation of each words: Aliquot Analyte Aqueous Calibration curve Composition sample Decantarrow_forward
- Use a suitable model to explain how separation and identification of a mixture of organic compounds can be achieved with a thin layer chromatographic (TLC) technique.arrow_forwardQUESTION 3 What is one disadvantage of using column chromatography? It is relatively ineffective at separating most organic compounds The desired compound must be the ≥95% majority compound in the initial mixture for a column to be effective It is only effective at separating room temperature gasses It wastes a significant amount of solventarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY