
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
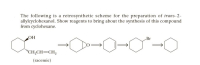
Transcribed Image Text:The following is a retrosynthetic scheme for the preparation of trans-2-
allylcyclohexanol. Show reagents to bring about the synthesis of this compound
from cyclohexane.
OH
Br
"CH,CH=CH,
(racemic)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- please help me draw thisarrow_forwardThe southern pine beetle utilizes a multi-component aggregation pheromone (one component shown below) to start mass colonization of healthy trees. The biosynthetic pathway involves the cyclization of this acetal from the straight chain structure. Draw the straight chain structure that could be used to form this acetal. Use wedges and dashes to correctly depict the stereochemistry. H3C- OH ✔arrow_forwardComplete the following reaction scheme. Keep in mind that the sequence of reagents converts an alcohol with 5 carbons or fewer to these precursors. Na,Cr,O, H2SO4, HO E Part 1 of 4 HBr C D A ㅏㅏ B NH3 Draw the structure for compound A and compound B.arrow_forward
- Sorbitol is a sweetener often used in chewing gum and diet drinks. It is obtained by the following reaction. Which of the following functional group transformations occur during this reaction? СНО CH2OH Н-с-ОН Н-с-ОН Но-с-н Н2 Но-с-н Н-с-ОН Н-с-оН Н-с-ОН н-с-он CH2OH CH-Он glucose sorbitol a. The aldehyde becomes an alcohol. b. An alcohol becomes an aldehyde. c. An alcohol disappears. d. A new aldehyde appears. e. All of the abovearrow_forwardcomplete these reactions with reagents and intermediates.arrow_forwardWhich of the following reagents would lead to a product that has two functional groups: an alcohol and an ether? NaOH/H₂O H3O+/H₂O HC1 CH3CH₂OH/CH3CH₂O™¹Na H₂C CH₂arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY