
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
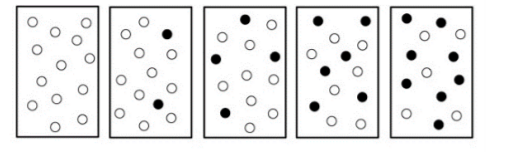
The following diagrams represent a hypothetical reaction A ⇋ B, with A represented by white spheres and B represented by black spheres. The sequence from left to right represents the system as time passes.
a. Do the diagrams indicate that the system has reached equilibrium? (Circle one) ( Yes / No )
b. Use concentration and reaction rate to describe what is occurring when a system has reached dynamic equilibrium.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements is FALSE regarding reactions in dynamic equilibrium? O a. At equilibrium, the rates of reactant and product formation are equal. O b. If a chemical species is removed in the system, the system will favor the reaction that forms back the removed species. O C. At equilibrium, the concentrations of all species are equal. O d. In an equilibrium system with Keg > 1, the [products]eg is greater than the [reactantsleg.arrow_forward|The three reaction systems (1, 2, and 3) depicted in the accompanying illustration can all be described by the equation 2A=B, where the blue circles are A and the purple ovals are B. Each set of panels shows the changing composition of one of the three reaction mixtures as a function of time. Which system took the longest to reach chemical equilibrium? t2 reaction system 1 reaction system 2 reaction system 3arrow_forwardno need to explain plsarrow_forward
- Use the given equilibrium constant to identify it's corresponding chemical equation assuming every reactant and product are in the gas phase. Keq = ([NH3]4[O2]3)/([N2]2[H2O]6)arrow_forwardWhich is the appropriate description to show the effect of a catalyst on the reaction rate and equilibrium in a reversible reaction: Reactants › Products O• The rate of the forward reaction is increased. • The rate of the reverse reaction is decreased 0 •The equilibrium position is displaced to the right O • The rate of the forward reaction is increased. The rate of the reverse reaction is increased The equilibrium position is unchanged. • The rate of the forward reaction is increased. 0 •The rate of the reverse reaction is unchanged • The equilibrium position is displaced to the right • The rate of the forward reaction is unchanged. The rate of the reverse reaction is unchanged The equilibrium position is unchanged. Suppose that an exothermic reaction, Reactants < › Products, is at equilibrium. According to Le Chatelier's Principle, if the reaction temperature is increased, in which direction will the equilibrium be displaced? O The equilibrium will be displaced toward the…arrow_forward7. Given the following equilibrium systems, note the direction the equilibrium will shift [→,-, NC, no change] when the named "stress" is applied. 2 NH3(g) + 92.5 kJ a 1 2 3 4 5 6 7 Stress Remove N2 (g) Add NH3 (9) Remove H2 (9) Remove NH3 (g) Add O2 (g) Decrease Pressure Add Heat N2(g) + 3 H2 (g) Equilibrium Shiftarrow_forward
- Consider the reaction. NaC2O2H3(s) <--- H2O ------> NaC2O2H3(aq), which is at equilibrium in an open flask in the lab at room temperature. You add more water to the equilibrium mixture. When the system reestablishes equilibrium, what has changed? a. the concentration of NaC2H3O2(aq) does not change b. heat is produced c. the concentration of NaC2H3O2(aq) decreases d. the concentration of the water increasesarrow_forward1. The reaction shown below is carried out at various temperatures and the value of Kc determined. Draw particulate representations that show the relative amounts of each reactant and product that would roughly correspond to the value of K. Legend = PC13 = Cl₂ ∞ = = PC15 PC13 (g) + Cl2 (g) = PC15 (g) Magnitude of K Particulate diagram of equilibrium mixture 1 x 10 -3 1 x 105arrow_forwardDecide whether each of the following statements is true or false. If false, change the wording of the statement to make it true. a) The magnitude of the equilibrium constant is always independent of temperature. b) When two chemical equations are added to give a net equation, the equilibrium constant for the net equation is the product of the equilibrium constants of the summed equations. c) The equilibrium constant for a reaction has the same value as K for the reverse reaction. d) Only the concentration of CO2 appears in the equilibrium expression for the reaction: CaCO3 (s) ↔ CaO (s) + CO2 (g). e) For the reaction CaCO3 (s) ↔ CaO (s) + CO2 (g), the value of K is numerically the same whether the amount of CO2 is expressed as molarity or as gas pressure.arrow_forward
- Label the following statements as true or false. 1) If Kc=5 for the reaction A + B <--> AB, then AB <--> A + B has a Kc=0.2 2) When a system is at equilibrium, the reactants and the products of the reaction will be equal in concentration. When Q > Kc, the reaction shifts to the left to reduce stress on the system. 3) When a system is at equilibrium, the reaction rate of the forward reaction is less than to the reaction rate of the reverse reaction. 4) When Q < Kc, the reaction shifts to the left to reduce stress on the system. 5) For a reversible reaction, the ΔH of the forward reaction is equal in magnitude but opposite in sign to the ΔH of the reverse reaction.arrow_forwardQUESTION 4 Adding a catalyst to an equilibrium system will: produce more products at equilibrium. produce more reactants at equilibrium. not change the amount of reactants or products at equilibrium.arrow_forwardWhen a system is a dynamic equilibrium, A no reactions are occurring B a reaction is occurring in only one direction C the rates of the forward and reverse reactions are equal D all of the reactants have been converted to productsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY