
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
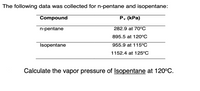
Transcribed Image Text:The following data was collected for n-pentane and isopentane:
Compound
P, (kPa)
n-pentane
282.9 at 70°C
895.5 at 120°C
Isopentane
955.9 at 115°C
1152.4 at 125°C
Calculate the vapor pressure of Isopentane at 120°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1) 1 mole of a monatomic ideal gas initially at 70 F and 0.83 atm is cooled; to 30 *F by placing the gas in thermal contact with the surroundings at 30 F (a) Calculate W, Q,AU, and AH in Joules if the cooling takes place at constant pressure. (b) Calculate W,Q, AU, and AH in Joules if the cooling takes place; at a constant volume.arrow_forward4. A mixture of 1 mole CO, and 3.5 moles of air is contained in a vessel at 1 bar and 15°C. The volumetric analysis of air can be taken as 21% oxygen and 79% nitrogen. Calculate for the mixture : (i) The masses of CO, 0, and N, and the total mass. (ii) The percentage carbon content by mass. (iii) The apparent molecular weight and the gas constant for the mixture. (iv) The specific volume of the mixture. [Ans. (i) 44 kg, 23.55 kg, 77.5 kg ; (ii) 8.27% ; (iii) 32.2, 0.2581 kJ/kg K ; (iv) 0.7435 m³/kg] na ofarrow_forward26. Please help me answer all parts to this physics questionarrow_forward
- Calculate the electricity energy required in units of D for desalinating 150 w of water and it takes 3.70 kWh/m^3 of water?arrow_forwardAt 25°C temperature and 1 bar pressure, one mole of gas is heated to 300°C in a piston-cylinder mechanism and pressurized to 10 bar pressure. Calculate the heat and work information required for this process for both methods below: Method A. Isothermal compression to 10 bar followed by isobaric process heating to 300°C. Method B. The PV will be compressed to constant, followed by isobaric process cooling or heating to 300°C if necessary. The gas can be assumed to be ideal (Cp = 38 J/(mol K). Solve correctly pleasearrow_forwardThermodynamics Question: The value of Henry's constant for 02 dissolved in water at 300 K is 45,000 bar. Water is exposed to air containing 20% 02 at pressure 100 kPa. What is the mole fraction of 02 in the water?arrow_forward
- 24. Compound A and compound B form an ideal liquid solution. The mole fraction of compound A in the solution is 0.30. The total pressure or vapor pressure above the solution is 94.6 mmHg. The vapor pressure of the pure compound A is 173 mmHg. Calculate the vapor pressure of the pure compound B. Ans: 61.0 mmHgarrow_forwardUse the data from this table of thermodynamic properties to calculate the values of AS for each of the reactions at 25 °C. C(s, graphite) + H, O(g) CO(g)+ H,(g) AS = N, (g) + 3 H, (g) - 2 NH,(g) AS = K Question Source. McQuararrow_forwardWhat is the temperature of the mixture if 0.6 kg of alcohol at 60 0C is added to 0,5 kg alcohol at 20 0C in polysterene cup?arrow_forward
- use JANAF tables from internetarrow_forwardFor methyl choride at 100 °C the virial coefficients are B= -242.5 cm3/mol and C= 25,000 cm/mol?. Calculate the work of a mechanically reversible, compression of I mol of methyl chloride from 1 bar to 55 bar at 00°C. Base calculations on the following farms of the virial equations: a. Z =1+(B/V)+(C/V²) b.Z =1+ B'P + C'P2 where B' = B/and C' = (C-B'²)/(RT)² %3D %3Darrow_forward16. (Solution Needed) An unknown compound is at 200°C and 1 MPa. What is the maximum number of phases that could be present? d. not possible to specify both temperature and pressure а. b. 1 е. 3 с. 4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY